当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stripped Charge of Ag less than Deposited one Owing to Negative Capacitance Caused by Redox Reactions
Electroanalysis ( IF 2.7 ) Pub Date : 2019-07-22 , DOI: 10.1002/elan.201800873 Koichi Jeremiah Aoki 1 , Jingyuan Chen 2 , Ru Wang 2
Electroanalysis ( IF 2.7 ) Pub Date : 2019-07-22 , DOI: 10.1002/elan.201800873 Koichi Jeremiah Aoki 1 , Jingyuan Chen 2 , Ru Wang 2
Affiliation
|
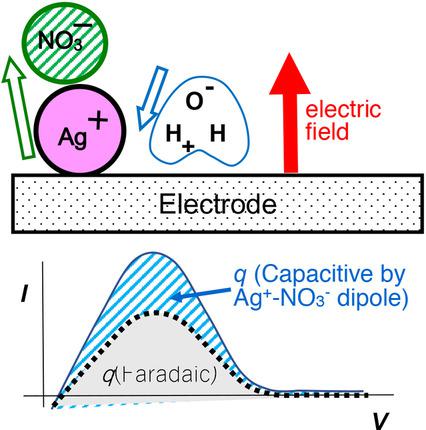
Anodic stripping voltammetry was made in AgNO3 solution, here Ag was deposited under long term potentiostatic conditions to evaluate the reduction charge, qr, and then was stripped by linear sweep voltammetry to determine the oxidation charge, qo. The charges were unbalanced, satisfying ca. qo=0.7|qr|, where other possible reduction charge such as by dioxygen and dichlorosilver were subtracted. The 30 % loss of the anodic charge can be ascribed to the negative capacitance by the potential sweep generation of Ag+. The generated Ag+ forms a dipole with a counter ion, of which orientation is the same as the direction of the externally applied electric field and opposite to the dipoles of solvent. The redox dipole decreases the conventional double layer capacitance caused by solvent dipoles, and high concentrations of Ag+ takes the capacitance to be negative values. The unbalanced charge, however, has no influence on quantitative determination of concentrations Ag+ by use of a calibration line.
中文翻译:

由于氧化还原反应引起的负电容,Ag的剥离电荷少于沉积的电荷
在AgNO 3溶液中进行阳极溶出伏安法,此处将Ag放置在长期恒电位条件下以评估还原电荷q r,然后通过线性扫描伏安法进行溶出以确定氧化电荷q o。收费是不平衡的,满足约。q o = 0.7 | q r |,其中减去其他可能的还原电荷,例如通过双氧和二氯银。阳极电荷的30%损耗可以归因于Ag +的电势扫描产生的负电容。生成的Ag +形成具有抗衡离子的偶极子,其方向与外部施加的电场的方向相同,并且与溶剂的偶极子相反。氧化还原偶极子减小了由溶剂偶极子引起的常规双层电容,并且高浓度的Ag +使电容为负值。但是,不平衡电荷对使用校准线定量测定Ag +的浓度没有影响。
更新日期:2019-11-18
中文翻译:

由于氧化还原反应引起的负电容,Ag的剥离电荷少于沉积的电荷
在AgNO 3溶液中进行阳极溶出伏安法,此处将Ag放置在长期恒电位条件下以评估还原电荷q r,然后通过线性扫描伏安法进行溶出以确定氧化电荷q o。收费是不平衡的,满足约。q o = 0.7 | q r |,其中减去其他可能的还原电荷,例如通过双氧和二氯银。阳极电荷的30%损耗可以归因于Ag +的电势扫描产生的负电容。生成的Ag +形成具有抗衡离子的偶极子,其方向与外部施加的电场的方向相同,并且与溶剂的偶极子相反。氧化还原偶极子减小了由溶剂偶极子引起的常规双层电容,并且高浓度的Ag +使电容为负值。但是,不平衡电荷对使用校准线定量测定Ag +的浓度没有影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号