当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decentralized Care with Generic Direct-Acting Antivirals in the Management of Chronic Hepatitis C in a Public Health Care Setting
Journal of Hepatology ( IF 26.8 ) Pub Date : 2019-12-01 , DOI: 10.1016/j.jhep.2019.07.006 Radha K Dhiman 1 , Gagandeep S Grover 2 , Madhumita Premkumar 3 , Sunil Taneja 3 , Ajay Duseja 3 , Sanjeev Arora 4 , Sahaj Rathi 3 , Sandeep Satsangi 3 , Akash Roy 3 ,
Journal of Hepatology ( IF 26.8 ) Pub Date : 2019-12-01 , DOI: 10.1016/j.jhep.2019.07.006 Radha K Dhiman 1 , Gagandeep S Grover 2 , Madhumita Premkumar 3 , Sunil Taneja 3 , Ajay Duseja 3 , Sanjeev Arora 4 , Sahaj Rathi 3 , Sandeep Satsangi 3 , Akash Roy 3 ,
Affiliation
|
BACKGROUND AND AIMS
The prevalence of anti-hepatitis C virus antibody in Punjab, India is 3.6% with an estimated 728,000 persons with viremic chronic hepatitis C (CHC). The Mukh-Mantri Punjab Hepatitis C Relief Fund, launched on 18th June 2016, provides no-cost generic direct-acting antivirals (DAAs) with sofosbuvir+ledipasvir±ribavirin or sofosbuvir+daclatasvir±ribavirin with the goal to eliminate CHC from Punjab. We assessed the safety and efficacy of decentralized treatment of CHC in a public health care setting. METHODS
Primary care providers from 3 University and 22 District Hospitals were trained to provide algorithm based DAAs treatment and supervised by telehealth clinics conducted fortnightly. The diagnosis of cirrhosis was based on clinical and radiological evidence, including AST-to-platelet ratio index (APRI ≥2.0) and FIB-4 score (>3.25) or on liver stiffness measurement ≥12.5 kPa on Fibroscan. RESULTS
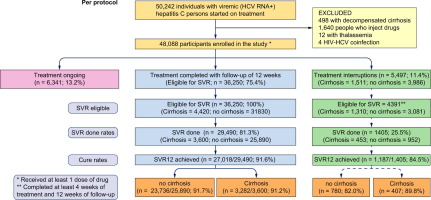
We enrolled 48,088 persons with CHC (63.8% male; mean age 42.1 years; 80.5% rural; 14.8% compensated cirrhosis; 69.9% genotype[G]3) between 18thJune2016 to 31stJuly2018. While 36,250 (75.4%) persons completed treatment, 5497 (11.4%) had treatment interruptions and 6341 (13.2%) persons are currently ongoing treatment. SVR-12 was achieved in 91.6% of persons per protocol, 67.6% in intention to treat (ITT) analysis where all interruptions were treated as failures and 91.2% in a modified ITT analysis where all persons with successful SVR-12 in the interruptions arm were included as cured. SVR-12 rates in persons with and without cirrhosis and G3 versus non-G3 were comparable. SVR-12 was 84.4% of persons who had treatment interruptions. CONCLUSION
Decentralized care of CHC with generic all oral DAA regimens is safe and effective regardless of genotype or presence of cirrhosis. Lay Summary.
中文翻译:

在公共医疗机构中使用通用直接作用抗病毒药物管理慢性丙型肝炎的分散护理
背景和目的 印度旁遮普邦抗丙型肝炎病毒抗体的流行率为 3.6%,估计有 728,000 人患有病毒血症性慢性丙型肝炎 (CHC)。Mukh-Mantri Punjab 丙型肝炎救助基金于 2016 年 6 月 18 日启动,提供免费的通用直接作用抗病毒药物 (DAA),包括索非布韦 + 来迪帕韦±利巴韦林或索非布韦 + daclatasvir±利巴韦林,旨在消除旁遮普的 CHC。我们评估了公共医疗机构中 CHC 分散治疗的安全性和有效性。方法 来自 3 所大学和 22 家地区医院的初级保健提供者接受了提供基于算法的 DAA 治疗的培训,并由每两周进行一次的远程医疗诊所进行监督。肝硬化的诊断基于临床和放射学证据,包括 AST 与血小板比率指数(APRI ≥ 2.0)和 FIB-4 评分(> 3.25) 或 Fibroscan 上肝脏硬度测量值≥12.5 kPa。结果 我们在 2016 年 6 月 18 日至 2018 年 7 月 31 日期间招募了 48,088 名 CHC 患者(63.8% 男性;平均年龄 42.1 岁;80.5% 农村;14.8% 代偿性肝硬化;69.9% 基因型 [G]3)。36,250 (75.4%) 人完成治疗,5,497 (11.4%) 人中断治疗,6,341 (13.2%) 人目前正在接受治疗。每个方案有 91.6% 的人实现了 SVR-12,在意向治疗 (ITT) 分析中 67.6% 的人实现了所有中断被视为失败,而在修改的 ITT 分析中 91.2% 的人在中断组中成功实现了 SVR-12被列为治愈。肝硬化和非肝硬化以及 G3 与非 G3 患者的 SVR-12 率相当。SVR-12 占治疗中断患者的 84.4%。结论 无论基因型或是否存在肝硬化,使用通用的全口服 DAA 方案对 CHC 进行分散治疗都是安全有效的。奠定总结。
更新日期:2019-12-01
中文翻译:

在公共医疗机构中使用通用直接作用抗病毒药物管理慢性丙型肝炎的分散护理
背景和目的 印度旁遮普邦抗丙型肝炎病毒抗体的流行率为 3.6%,估计有 728,000 人患有病毒血症性慢性丙型肝炎 (CHC)。Mukh-Mantri Punjab 丙型肝炎救助基金于 2016 年 6 月 18 日启动,提供免费的通用直接作用抗病毒药物 (DAA),包括索非布韦 + 来迪帕韦±利巴韦林或索非布韦 + daclatasvir±利巴韦林,旨在消除旁遮普的 CHC。我们评估了公共医疗机构中 CHC 分散治疗的安全性和有效性。方法 来自 3 所大学和 22 家地区医院的初级保健提供者接受了提供基于算法的 DAA 治疗的培训,并由每两周进行一次的远程医疗诊所进行监督。肝硬化的诊断基于临床和放射学证据,包括 AST 与血小板比率指数(APRI ≥ 2.0)和 FIB-4 评分(> 3.25) 或 Fibroscan 上肝脏硬度测量值≥12.5 kPa。结果 我们在 2016 年 6 月 18 日至 2018 年 7 月 31 日期间招募了 48,088 名 CHC 患者(63.8% 男性;平均年龄 42.1 岁;80.5% 农村;14.8% 代偿性肝硬化;69.9% 基因型 [G]3)。36,250 (75.4%) 人完成治疗,5,497 (11.4%) 人中断治疗,6,341 (13.2%) 人目前正在接受治疗。每个方案有 91.6% 的人实现了 SVR-12,在意向治疗 (ITT) 分析中 67.6% 的人实现了所有中断被视为失败,而在修改的 ITT 分析中 91.2% 的人在中断组中成功实现了 SVR-12被列为治愈。肝硬化和非肝硬化以及 G3 与非 G3 患者的 SVR-12 率相当。SVR-12 占治疗中断患者的 84.4%。结论 无论基因型或是否存在肝硬化,使用通用的全口服 DAA 方案对 CHC 进行分散治疗都是安全有效的。奠定总结。











































 京公网安备 11010802027423号
京公网安备 11010802027423号