当前位置:
X-MOL 学术
›
npj Precis. Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Blood-based monitoring identifies acquired and targetable driver HER2 mutations in endocrine-resistant metastatic breast cancer.
npj Precision Oncology ( IF 6.8 ) Pub Date : 2019-07-16 , DOI: 10.1038/s41698-019-0090-5 Arielle J Medford 1, 2 , Taronish D Dubash 1 , Dejan Juric 1 , Laura Spring 1, 2 , Andrzej Niemierko 1 , Neelima Vidula 1, 2 , Jeffrey Peppercorn 1, 2 , Steven Isakoff 1, 2 , Brittany A Reeves 1 , Joseph A LiCausi 1 , Benjamin Wesley 1 , Giuliana Malvarosa 1 , Megan Yuen 1 , Ben S Wittner 1 , Michael S Lawrence 1 , A John Iafrate 1, 3 , Leif Ellisen 1, 2 , Beverly Moy 1, 2 , Mehmet Toner 4, 5 , Shyamala Maheswaran 1, 4 , Daniel A Haber 1, 2, 6 , Aditya Bardia 1, 2
npj Precision Oncology ( IF 6.8 ) Pub Date : 2019-07-16 , DOI: 10.1038/s41698-019-0090-5 Arielle J Medford 1, 2 , Taronish D Dubash 1 , Dejan Juric 1 , Laura Spring 1, 2 , Andrzej Niemierko 1 , Neelima Vidula 1, 2 , Jeffrey Peppercorn 1, 2 , Steven Isakoff 1, 2 , Brittany A Reeves 1 , Joseph A LiCausi 1 , Benjamin Wesley 1 , Giuliana Malvarosa 1 , Megan Yuen 1 , Ben S Wittner 1 , Michael S Lawrence 1 , A John Iafrate 1, 3 , Leif Ellisen 1, 2 , Beverly Moy 1, 2 , Mehmet Toner 4, 5 , Shyamala Maheswaran 1, 4 , Daniel A Haber 1, 2, 6 , Aditya Bardia 1, 2
Affiliation
|
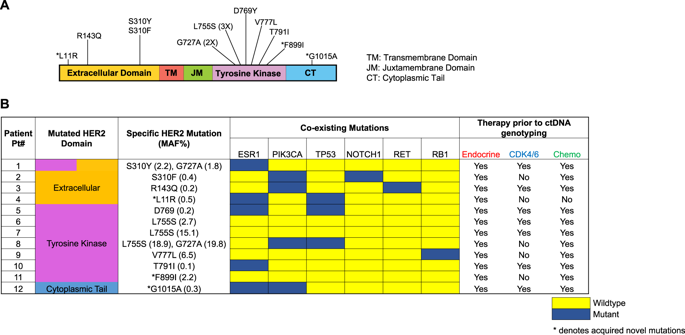
Plasma genotyping identifies potentially actionable mutations at variable mutant allele frequencies, often admixed with multiple subclonal variants, highlighting the need for their clinical and functional validation. We prospectively monitored plasma genotypes in 143 women with endocrine-resistant metastatic breast cancer (MBC), identifying multiple novel mutations including HER2 mutations (8.4%), albeit at different frequencies highlighting clinical heterogeneity. To evaluate functional significance, we established ex vivo culture from circulating tumor cells (CTCs) from a patient with HER2-mutant MBC, which revealed resistance to multiple targeted therapies including endocrine and CDK 4/6 inhibitors, but high sensitivity to neratinib (IC50: 0.018 μM). Immunoblotting analysis of the HER2-mutant CTC culture line revealed high levels of HER2 expression at baseline were suppressed by neratinib, which also abrogated downstream signaling, highlighting oncogenic dependency with HER2 mutation. Furthermore, treatment of an index patient with HER2-mutant MBC with the irreversible HER2 inhibitor neratinib resulted in significant clinical response, with complete molecular resolution of two distinct clonal HER2 mutations, with persistence of other passenger subclones, confirming HER2 alteration as a driver mutation. Thus, driver HER2 mutant alleles that emerge during blood-based monitoring of endocrine-resistant MBC confer novel therapeutic vulnerability, and ex vivo expansion of viable CTCs from the blood circulation may broadly complement plasma-based mutational analysis in MBC.
中文翻译:

基于血液的监测可识别内分泌耐药性转移性乳腺癌中获得性和可靶向的驱动 HER2 突变。
血浆基因分型可识别可变突变等位基因频率下潜在的可操作突变,这些突变通常与多个亚克隆变体混合,突出了对其临床和功能验证的需要。我们前瞻性监测了 143 名患有内分泌抵抗性转移性乳腺癌 (MBC) 的女性的血浆基因型,发现了多种新突变,包括 HER2 突变 (8.4%),尽管频率不同,突出了临床异质性。为了评估功能意义,我们对 HER2 突变 MBC 患者的循环肿瘤细胞 (CTC) 进行了离体培养,结果显示对包括内分泌和 CDK 4/6 抑制剂在内的多种靶向治疗具有耐药性,但对来那替尼具有高敏感性(IC50: 0.018μM)。对 HER2 突变 CTC 培养系的免疫印迹分析显示,基线时高水平的 HER2 表达被来那替尼抑制,这也消除了下游信号传导,突出了 HER2 突变的致癌依赖性。此外,用不可逆 HER2 抑制剂 neratinib 治疗 HER2 突变 MBC 指标患者产生了显着的临床反应,两种不同克隆 HER2 突变得到完全分子解决,并且其他过客亚克隆持续存在,证实 HER2 改变是驱动突变。因此,在内分泌耐药 MBC 的血液监测过程中出现的驱动 HER2 突变等位基因赋予了新的治疗脆弱性,并且从血液循环中离体扩增活 CTC 可能广泛补充 MBC 中基于血浆的突变分析。
更新日期:2019-07-16
中文翻译:

基于血液的监测可识别内分泌耐药性转移性乳腺癌中获得性和可靶向的驱动 HER2 突变。
血浆基因分型可识别可变突变等位基因频率下潜在的可操作突变,这些突变通常与多个亚克隆变体混合,突出了对其临床和功能验证的需要。我们前瞻性监测了 143 名患有内分泌抵抗性转移性乳腺癌 (MBC) 的女性的血浆基因型,发现了多种新突变,包括 HER2 突变 (8.4%),尽管频率不同,突出了临床异质性。为了评估功能意义,我们对 HER2 突变 MBC 患者的循环肿瘤细胞 (CTC) 进行了离体培养,结果显示对包括内分泌和 CDK 4/6 抑制剂在内的多种靶向治疗具有耐药性,但对来那替尼具有高敏感性(IC50: 0.018μM)。对 HER2 突变 CTC 培养系的免疫印迹分析显示,基线时高水平的 HER2 表达被来那替尼抑制,这也消除了下游信号传导,突出了 HER2 突变的致癌依赖性。此外,用不可逆 HER2 抑制剂 neratinib 治疗 HER2 突变 MBC 指标患者产生了显着的临床反应,两种不同克隆 HER2 突变得到完全分子解决,并且其他过客亚克隆持续存在,证实 HER2 改变是驱动突变。因此,在内分泌耐药 MBC 的血液监测过程中出现的驱动 HER2 突变等位基因赋予了新的治疗脆弱性,并且从血液循环中离体扩增活 CTC 可能广泛补充 MBC 中基于血浆的突变分析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号