Cancer Gene Therapy ( IF 6.4 ) Pub Date : 2019-07-16 , DOI: 10.1038/s41417-019-0125-7 Duanrui Liu 1 , Xiaoli Ma 1 , Fei Yang 2 , Dongjie Xiao 1 , Yanfei Jia 1, 3 , Yunshan Wang 1, 3
|
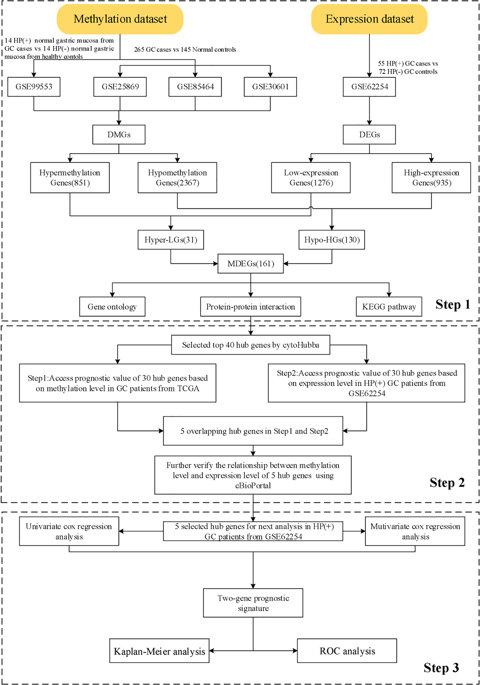
DNA methylation has an important role in Helicobacter pylori (H. pylori)-induced gastric cancer (GC) processes and development. The aim of this study was to search genome-scale epigenetic modifications for studying pathogenesis of H. pylori-induced GC, and to find factors and powerful signature related to survival and prognosis. In this study, we conducted a comprehensive analysis of DNA methylation and gene expression profiles in the Gene Expression Omnibus (GEO), to identified differentially expressed genes (DEGs) and differentially methylated genes (DMGs). Functional enrichment analysis of the screened genes was performed, and a protein–protein interaction network was constructed. The TCGA DNA methylation databases and 55 H. pylori-infected GC cases of GEO RNA sequencing (GSE62254) were utilized for prognostic value validation of hub genes. Finally, a prognosis-related risk signature was identified by a series of bioinformatics analysis for H. pylori-induced GC patients. Totally, 161 DMGs were identified. Pathway analysis showed that all MDEGs mainly associated with Ras signaling pathway, renal cell carcinoma, mitogen-activated protein kinase signaling pathway. Five hub genes including CACNB2, GNB4, GRIN2A, MEF2C, and PREX1 were screened as independent prognostic factors in H. pylori-induced GC patients. Two-gene (CACNB2 and MEF2C) risk signature was constructed for predicting the overall survival of H. pylori-induced GC patients. Our study indicated possible MDEGs and pathways in H. pylori-induced GC by bioinformatics analysis, which may provide novel insights for unraveling pathogenesis of H. pylori-induced GC. Hub genes might serve as aberrantly methylation-based biomarkers for clinical diagnostic and prognostic evaluation of H. pylori-induced GC.
中文翻译:

幽门螺杆菌诱导的胃癌中甲基化差异表达基因的发现和验证。
DNA 甲基化在幽门螺杆菌( H. pylori ) 诱导的胃癌 (GC) 过程和发展中具有重要作用。本研究的目的是寻找基因组规模的表观遗传修饰,以研究幽门螺杆菌诱导的 GC 的发病机制,并寻找与生存和预后相关的因素和强大的特征。在这项研究中,我们对基因表达综合 (GEO) 中的 DNA 甲基化和基因表达谱进行了全面分析,以识别差异表达基因 (DEG) 和差异甲基化基因 (DMG)。对筛选出的基因进行功能富集分析,构建蛋白质-蛋白质相互作用网络。TCGA DNA 甲基化数据库和 55 H. pyloriGEO RNA 测序 (GSE62254) 的感染 GC 病例被用于中心基因的预后价值验证。最后,通过对幽门螺杆菌诱导的 GC 患者的一系列生物信息学分析,确定了与预后相关的风险特征。共识别出 161 个 DMG。通路分析显示所有MDEGs主要与Ras信号通路、肾细胞癌、丝裂原活化蛋白激酶信号通路相关。包括 CACNB2、GNB4、GRIN2A、MEF2C 和 PREX1 在内的五个中枢基因被筛选为幽门螺杆菌诱导的 GC 患者的独立预后因素。构建了双基因(CACNB2 和 MEF2C)风险特征以预测幽门螺杆菌诱导的 GC 患者的总体存活率。我们的研究表明可能的 MDEGs 和途径通过生物信息学分析H. pylori诱导的 GC,这可能为阐明H. pylori诱导的 GC 的发病机制提供新的见解。Hub 基因可能作为异常甲基化的生物标志物,用于幽门螺杆菌诱导的 GC 的临床诊断和预后评估。



























 京公网安备 11010802027423号
京公网安备 11010802027423号