当前位置:
X-MOL 学术
›
Nutr. Diabetes
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a delayed-release nutrient for appetite control in adults with obesity and type 2 diabetes and initial clinical testing in a single dose randomized controlled trial.
Nutrition & Diabetes ( IF 4.6 ) Pub Date : 2019-07-15 , DOI: 10.1038/s41387-019-0088-7 E Beale 1 , E Lim 2 , H Yassine 1 , C Azen 2 , C Christopher 3
Nutrition & Diabetes ( IF 4.6 ) Pub Date : 2019-07-15 , DOI: 10.1038/s41387-019-0088-7 E Beale 1 , E Lim 2 , H Yassine 1 , C Azen 2 , C Christopher 3
Affiliation
|
BACKGROUND AND OBJECTIVES
Delivery of nutrients directly to the small intestine, either via enteral feeding tube or by gastric bypass surgery, is associated with increased levels of appetite-suppressing and glucoregulatory hormones, including GLP-1, and reduced appetite. Achieving these changes non-invasively using formulated foods may be of therapeutic benefit in individuals with obesity and related comorbidities. The aim of this pilot study was to determine the effect of a single dose of a novel delayed-release nutrient (DRN) on glucose, GLP-1, c-peptide, insulin, and appetite in adults with obesity and type 2 diabetes.
SUBJECTS AND METHODS
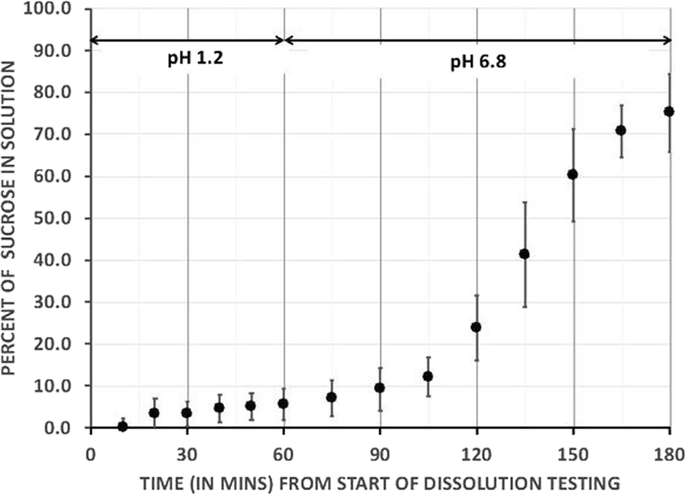
We formulated an all-natural, generally recognized as safe ('GRAS") DRN and conducted a randomized prospective crossover trial. Nineteen adults with obesity and type 2 diabetes underwent paired 3-h meal tolerance tests (MTT) in randomized order 1-4 weeks apart. Subjects ingested a single dose of DRN and the same nutrients as unformulated powders (UN).
RESULTS
For DRN compared with UN, the maximal concentration (Cmax) was significantly lower for glucose, c-peptide, and insulin, and the time of maximal concentration (Tmax) was significantly delayed. While Tmax for GLP-1 was also significantly delayed following DRN compared with UN (45 min later; p = 0.26), Cmax did not differ significantly. GLP-1 rose significantly during the last 90 min of the 3-h MTT (β1 = 0.16 pg/mL/min, p = 0.025), while following UN it decreased (β1 = -0.21 pg/mL/min, p = 0.0026) (p difference = 0.0003). There were minimal differences in seven measures of appetite and adverse symptoms between DRN and UN.
CONCLUSIONS
We conclude that nutrient can be formulated using all-natural ingredients to induce a delayed rise in GLP-1. Further testing is needed to determine the amount and site of nutrient release, when maximum GLP-1 levels occur, and if modification of the formulation specifications and dose are associated with appetite and glucose control.
中文翻译:

在肥胖和2型糖尿病的成年人中开发用于控制食欲的延迟释放营养素,并在单剂量随机对照试验中进行初始临床测试。
背景和目的通过肠内饲管或通过胃旁路手术将营养物质直接输送到小肠,会增加食欲抑制和糖调节激素(包括GLP-1)的水平,并降低食欲。使用配制食品以非侵入方式实现这些改变可能对肥胖症和相关合并症患者具有治疗益处。这项初步研究的目的是确定单剂量新型延迟释放营养素(DRN)对肥胖和2型糖尿病成年人的葡萄糖,GLP-1,c肽,胰岛素和食欲的影响。受试者和方法我们制定了一种全天然的,公认的安全('GRAS')DRN,并进行了一项随机的前瞻性交叉试验。十九位肥胖和2型糖尿病成年人以1-4周的随机顺序接受了配对的3小时耐餐性测试(MTT)。受试者摄入单剂量的DRN和与非配方散剂(UN)相同的营养素。结果对于DRN,与UN相比,葡萄糖,c肽和胰岛素的最大浓度(Cmax)明显降低,最大浓度(Tmax)的时间明显延迟。虽然DLP后GLP-1的Tmax也比UN显着延迟(45分钟后; p = 0.26),但Cmax并没有显着差异。在3小时MTT的最后90分钟内,GLP-1显着上升(β1= 0.16 pg / mL / min,p = 0.025),而在联合国之后,GLP-1下降(β1= -0.21 pg / mL / min,p = 0.0026) )(p差异= 0.0003)。DRN与联合国在7种食欲和不良症状指标上的差异极小。结论我们得出结论,可以使用全天然成分配制营养物质,以诱导GLP-1的延迟上升。当最大的GLP-1水平出现时,以及配方规格和剂量的改变是否与食欲和葡萄糖控制有关时,需要进一步测试以确定养分释放的数量和部位。
更新日期:2019-07-15
中文翻译:

在肥胖和2型糖尿病的成年人中开发用于控制食欲的延迟释放营养素,并在单剂量随机对照试验中进行初始临床测试。
背景和目的通过肠内饲管或通过胃旁路手术将营养物质直接输送到小肠,会增加食欲抑制和糖调节激素(包括GLP-1)的水平,并降低食欲。使用配制食品以非侵入方式实现这些改变可能对肥胖症和相关合并症患者具有治疗益处。这项初步研究的目的是确定单剂量新型延迟释放营养素(DRN)对肥胖和2型糖尿病成年人的葡萄糖,GLP-1,c肽,胰岛素和食欲的影响。受试者和方法我们制定了一种全天然的,公认的安全('GRAS')DRN,并进行了一项随机的前瞻性交叉试验。十九位肥胖和2型糖尿病成年人以1-4周的随机顺序接受了配对的3小时耐餐性测试(MTT)。受试者摄入单剂量的DRN和与非配方散剂(UN)相同的营养素。结果对于DRN,与UN相比,葡萄糖,c肽和胰岛素的最大浓度(Cmax)明显降低,最大浓度(Tmax)的时间明显延迟。虽然DLP后GLP-1的Tmax也比UN显着延迟(45分钟后; p = 0.26),但Cmax并没有显着差异。在3小时MTT的最后90分钟内,GLP-1显着上升(β1= 0.16 pg / mL / min,p = 0.025),而在联合国之后,GLP-1下降(β1= -0.21 pg / mL / min,p = 0.0026) )(p差异= 0.0003)。DRN与联合国在7种食欲和不良症状指标上的差异极小。结论我们得出结论,可以使用全天然成分配制营养物质,以诱导GLP-1的延迟上升。当最大的GLP-1水平出现时,以及配方规格和剂量的改变是否与食欲和葡萄糖控制有关时,需要进一步测试以确定养分释放的数量和部位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号