Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial.
The Lancet ( IF 168.9 ) Pub Date : 2019-07-13 , DOI: 10.1016/s0140-6736(19)31606-x Robert Croop 1 , Peter J Goadsby 2 , David A Stock 1 , Charles M Conway 1 , Micaela Forshaw 1 , Elyse G Stock 1 , Vladimir Coric 1 , Richard B Lipton 3
中文翻译:

瑞格非特口腔崩解片治疗偏头痛的疗效,安全性和耐受性:一项随机,3期,双盲,安慰剂对照试验。
更新日期:2019-08-30
The Lancet ( IF 168.9 ) Pub Date : 2019-07-13 , DOI: 10.1016/s0140-6736(19)31606-x Robert Croop 1 , Peter J Goadsby 2 , David A Stock 1 , Charles M Conway 1 , Micaela Forshaw 1 , Elyse G Stock 1 , Vladimir Coric 1 , Richard B Lipton 3
Affiliation
|
Background
Rimegepant, a small molecule calcitonin gene-related peptide receptor antagonist, has shown efficacy in the acute treatment of migraine using a standard tablet formulation. The objective of this trial was to compare the efficacy, safety, and tolerability of a novel orally disintegrating tablet formulation of rimegepant at 75 mg with placebo in the acute treatment of migraine.Methods
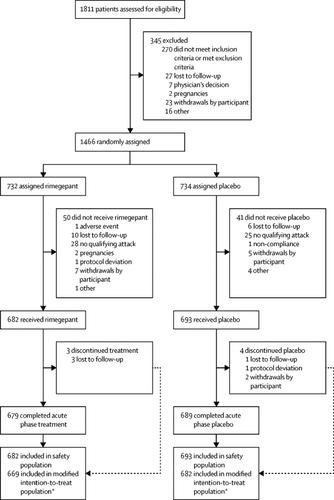
In this double-blind, randomised, placebo-controlled, multicentre phase 3 trial, adults aged 18 years or older with history of migraine of at least 1 year were recruited to 69 study centres in the USA. Participants were randomly assigned to receive rimegepant (75 mg orally disintegrating tablet) or placebo and instructed to treat a single migraine attack of moderate or severe pain intensity. The randomisation was stratified by the use of prophylactic medication (yes or no), and was carried out using an interactive web response system that was accessed by each clinical site. All participants, investigators, and the sponsor were masked to treatment group assignment. The coprimary endpoints were freedom from pain and freedom from the most bothersome symptom at 2 h postdose. The efficacy analyses used the modified intention-to-treat population, which included all patients who were randomly assigned, had a migraine attack with pain of moderate or severe intensity, took a dose of rimegepant or placebo, and had at least one efficacy assessment after administration of the dose. The safety analyses included all randomly assigned participants who received at least one dose of study medication. This study is registered with , number , and is closed to accrual.Findings
Between Feb 27 and Aug 28, 2018, 1811 participants were recruited and assessed for eligibility. 1466 participants were randomly assigned to the rimegepant (n=732) or placebo (n=734) groups, of whom 1375 received treatment with rimegepant (n=682) or placebo (n=693), and 1351 were evaluated for efficacy (rimegepant n=669, placebo n=682). At 2 h postdose, rimegepant orally disintegrating tablet was superior to placebo for freedom from pain (21% vs 11%, p<0·0001; risk difference 10, 95% CI 6–14) and freedom from the most bothersome symptom (35% vs 27%, p=0·0009; risk difference 8, 95% CI 3–13). The most common adverse events were nausea (rimegepant n=11 [2%]; placebo n=3 [<1%]) and urinary tract infection (rimegepant n=10 [1%]; placebo n=4 [1%]). One participant in each treatment group had a transaminase concentration of more than 3 × the upper limit of normal; neither was related to study medication, and no elevations in bilirubin greater than 2 × the upper limit of normal were reported. Treated participants reported no serious adverse events.Interpretation
In the acute treatment of migraine, a single 75 mg dose of rimegepant in an orally disintegrating tablet formulation was more effective than placebo. Tolerability was similar to placebo, with no safety concerns.Funding
Biohaven Pharmaceuticals.中文翻译:

瑞格非特口腔崩解片治疗偏头痛的疗效,安全性和耐受性:一项随机,3期,双盲,安慰剂对照试验。



























 京公网安备 11010802027423号
京公网安备 11010802027423号