Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Treatment of rat thyrocytes in vitro with cathepsin B and L inhibitors results in disruption of primary cilia leading to redistribution of the trace amine associated receptor 1 to the endoplasmic reticulum.
Biochimie ( IF 3.9 ) Pub Date : 2019-07-11 , DOI: 10.1016/j.biochi.2019.07.010 Joanna Szumska 1 , Zaina Batool 1 , Alaa Al-Hashimi 1 , Vaishnavi Venugopalan 1 , Vladislav Skripnik 1 , Norbert Schaschke 2 , Matthew Bogyo 3 , Klaudia Brix 1
Biochimie ( IF 3.9 ) Pub Date : 2019-07-11 , DOI: 10.1016/j.biochi.2019.07.010 Joanna Szumska 1 , Zaina Batool 1 , Alaa Al-Hashimi 1 , Vaishnavi Venugopalan 1 , Vladislav Skripnik 1 , Norbert Schaschke 2 , Matthew Bogyo 3 , Klaudia Brix 1
Affiliation
|
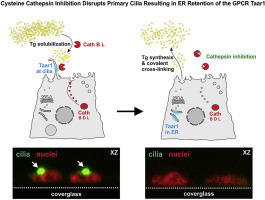
Taar1 is a G protein-coupled receptor (GPCR) confined to primary cilia of rodent thyroid epithelial cells. Taar1-deficient mouse thyroid follicles feature luminal accumulation of thyroglobulin suggesting that Taar1 acts as a regulator of extra- and pericellular thyroglobulin processing, which is mediated by cysteine cathepsin proteases present at the apical plasma membrane of rodent thyrocytes. Here, by immunostaining and confocal laser scanning microscopy, we demonstrated co-localization of cathepsin L, but only little cathepsin B, with Taar1 at primary cilia of rat thyrocytes, the FRT cells. Because proteases were shown to affect half-lives of certain receptors, we determined the effect of cathepsin activity inhibition on sub-cellular localization of Taar1 in FRT cells, whereupon Taar1 localization altered such that it was retained in compartments of the secretory pathway. Since the same effect on Taar1 localization was observed in both cathepsin B and L inhibitor-treated cells, the interaction of cathepsin activities and sub-cellular localization of Taar1 was thought to be indirect. Indeed, we observed that cathepsin inhibition resulted in a lack of primary cilia from FRT cells. Next, we proved that primary cilia are a necessity for Taar1 trafficking to reach the plasma membrane of FRT cells, since the disruption of primary cilia by treatment with β-cyclodextrin resulted in Taar1 retention in compartments of the secretory pathway. Furthermore, in less well-polarized rat thyrocytes, namely in FRTL-5 cells lacking primary cilia, Taar1 was mainly confined to the compartments of the secretory pathway. We conclude that Taar1 localization in polarized thyroid epithelial cells requires the presence of primary cilia, which is dependent on the proteolytic activity of cysteine cathepsins B and L.
中文翻译:

用组织蛋白酶B和L抑制剂体外处理大鼠甲状腺细胞会导致原发纤毛破裂,从而导致痕量胺相关受体1重新分布到内质网。
Taar1是一种G蛋白偶联受体(GPCR),局限于啮齿动物甲状腺上皮细胞的初级纤毛。Taar1缺陷的小鼠甲状腺滤泡具有甲状腺球蛋白的腔内积聚,表明Taar1充当细胞外和周围细胞甲状腺球蛋白加工的调节剂,这是由啮齿类甲状腺细胞顶质膜上存在的半胱氨酸组织蛋白酶所介导的。在这里,通过免疫染色和共聚焦激光扫描显微镜,我们证明了组织蛋白酶L,但只有很少的组织蛋白酶B与Taar1在大鼠甲状腺细胞(FRT细胞)的初级纤毛上共定位。由于显示蛋白酶会影响某些受体的半衰期,因此我们确定了组织蛋白酶活性抑制对FRT细胞中Taar1的亚细胞定位的影响,因此,Taar1的定位发生了变化,以使其保留在分泌途径的区室中。由于在组织蛋白酶B和L抑制剂处理的细胞中均观察到对Taar1定位的相同作用,因此组织蛋白酶活性与Taar1的亚细胞定位之间的相互作用被认为是间接的。实际上,我们观察到组织蛋白酶抑制导致FRT细胞缺乏初级纤毛。接下来,我们证明了原发纤毛是Taar1转运到达FRT细胞质膜的必要条件,因为用β-环糊精处理破坏原发纤毛会导致Taar1保留在分泌途径的隔室中。此外,在极化程度较低的大鼠甲状腺细胞中,即在缺乏原发纤毛的FRTL-5细胞中,Taar1主要局限于分泌途径的区室。
更新日期:2019-07-12
中文翻译:

用组织蛋白酶B和L抑制剂体外处理大鼠甲状腺细胞会导致原发纤毛破裂,从而导致痕量胺相关受体1重新分布到内质网。
Taar1是一种G蛋白偶联受体(GPCR),局限于啮齿动物甲状腺上皮细胞的初级纤毛。Taar1缺陷的小鼠甲状腺滤泡具有甲状腺球蛋白的腔内积聚,表明Taar1充当细胞外和周围细胞甲状腺球蛋白加工的调节剂,这是由啮齿类甲状腺细胞顶质膜上存在的半胱氨酸组织蛋白酶所介导的。在这里,通过免疫染色和共聚焦激光扫描显微镜,我们证明了组织蛋白酶L,但只有很少的组织蛋白酶B与Taar1在大鼠甲状腺细胞(FRT细胞)的初级纤毛上共定位。由于显示蛋白酶会影响某些受体的半衰期,因此我们确定了组织蛋白酶活性抑制对FRT细胞中Taar1的亚细胞定位的影响,因此,Taar1的定位发生了变化,以使其保留在分泌途径的区室中。由于在组织蛋白酶B和L抑制剂处理的细胞中均观察到对Taar1定位的相同作用,因此组织蛋白酶活性与Taar1的亚细胞定位之间的相互作用被认为是间接的。实际上,我们观察到组织蛋白酶抑制导致FRT细胞缺乏初级纤毛。接下来,我们证明了原发纤毛是Taar1转运到达FRT细胞质膜的必要条件,因为用β-环糊精处理破坏原发纤毛会导致Taar1保留在分泌途径的隔室中。此外,在极化程度较低的大鼠甲状腺细胞中,即在缺乏原发纤毛的FRTL-5细胞中,Taar1主要局限于分泌途径的区室。



























 京公网安备 11010802027423号
京公网安备 11010802027423号