当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxycodone-Mediated Activation of the Mu Opioid Receptor Reduces Whole Brain Functional Connectivity in Mice.
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-06-28 , DOI: 10.1021/acsptsci.9b00021 Md Taufiq Nasseef 1 , Jai Puneet Singh 1 , Aliza T Ehrlich 1 , Michael McNicholas 1 , Da Woon Park 1 , Weiya Ma 1 , Praveen Kulkarni 2 , Brigitte L Kieffer 1 , Emmanuel Darcq 1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-06-28 , DOI: 10.1021/acsptsci.9b00021 Md Taufiq Nasseef 1 , Jai Puneet Singh 1 , Aliza T Ehrlich 1 , Michael McNicholas 1 , Da Woon Park 1 , Weiya Ma 1 , Praveen Kulkarni 2 , Brigitte L Kieffer 1 , Emmanuel Darcq 1
Affiliation
|
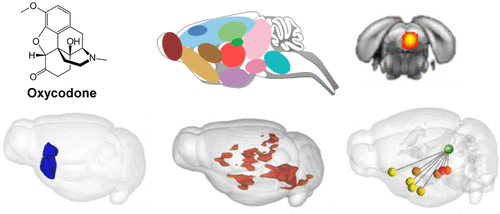
Oxycodone is a potent medicinal opioid analgesic to treat pain. It is also addictive and a main cause for the current opioid crisis. At present, the impact of oxycodone on coordinated brain network activities, and contribution of the mu opioid receptor (MOR) to these effects, is unknown. We used pharmacological magnetic resonance imaging in mice to characterize MOR-mediated oxycodone effects on whole-brain functional connectivity (FC). Control (CTL) and MOR knockout (KO) animals were imaged under dexmedetomidine in a 7Tesla scanner. Acquisition was performed continuously before and after 2 mg/kg oxycodone administration (analgesic in CTL mice). Independent component analysis (data-driven) produced a correlation matrix, showing widespread oxycodone-induced reduction of FC across 71 components. Isocortex, nucleus accumbens (NAc), pontine reticular nucleus, and periacqueducal gray (PAG) components showed the highest number of significant changes. Seed-to-voxel FC analysis (hypothesis-driven) was then focused on PAG and NAc considered key pain and reward centers. The two seeds showed reduced FC with 8 and 22 Allen Brain Atlas-based regions, respectively, in CTL but not KO mice. Further seed-to-seed quantification showed highest FC modifications of both PAG and NAc seeds with hypothalamic and amygdalar areas, as well as between them, revealing the strongest impact across reward and aversion/pain centers of the brain. In conclusion, we demonstrate that oxycodone reduces brain communication in a MOR-dependent manner, and establish a preliminary whole-brain FC signature of oxycodone. This proof-of-principle study provides a unique platform and reference data set to test other MOR opioid agonists and perhaps discover new mechanisms and FC biomarkers predicting safer analgesics.
中文翻译:

羟考酮介导的类阿片受体的激活减少了小鼠的全脑功能连通性。
羟考酮是一种有效的药用阿片类镇痛药,可用于治疗疼痛。它也是令人上瘾的,并且是当前阿片类药物危机的主要原因。目前,羟考酮对协调的脑网络活动的影响以及μ阿片受体(MOR)对这些作用的贡献尚不清楚。我们在小鼠中使用药理磁共振成像来表征MOR介导的羟考酮对全脑功能连接性(FC)的影响。对照(CTL)和MOR基因敲除(KO)动物在右美托咪定下在7Tesla扫描仪中成像。在施用2 mg / kg羟考酮之前和之后连续进行采集(在CTL小鼠中止痛)。独立成分分析(数据驱动)产生了相关矩阵,显示了羟考酮引起的71种成分中FC的广泛减少。等皮质,伏隔核(NAc),桥骨的网状细胞核和水囊周围的灰色(PAG)成分显示出最多的显着变化。种子到体素的FC分析(假设驱动)然后集中在PAG和NAc上,PAG和NAc被认为是关键的疼痛和奖励中心。在CTL小鼠中,这两个种子显示FC减少,FC减少了8个和22个基于Allen Brain Atlas的区域,而KO小鼠则没有。进一步的种子对种子定量显示,PAG和NAc种子具有下丘脑和杏仁核区域以及它们之间以及它们之间的FC修饰最高,从而揭示了大脑奖励和厌恶/疼痛中心的最强影响。总之,我们证明了羟考酮以MOR依赖的方式减少了大脑的交流,并建立了羟考酮的初步全脑FC签名。
更新日期:2019-07-11
中文翻译:

羟考酮介导的类阿片受体的激活减少了小鼠的全脑功能连通性。
羟考酮是一种有效的药用阿片类镇痛药,可用于治疗疼痛。它也是令人上瘾的,并且是当前阿片类药物危机的主要原因。目前,羟考酮对协调的脑网络活动的影响以及μ阿片受体(MOR)对这些作用的贡献尚不清楚。我们在小鼠中使用药理磁共振成像来表征MOR介导的羟考酮对全脑功能连接性(FC)的影响。对照(CTL)和MOR基因敲除(KO)动物在右美托咪定下在7Tesla扫描仪中成像。在施用2 mg / kg羟考酮之前和之后连续进行采集(在CTL小鼠中止痛)。独立成分分析(数据驱动)产生了相关矩阵,显示了羟考酮引起的71种成分中FC的广泛减少。等皮质,伏隔核(NAc),桥骨的网状细胞核和水囊周围的灰色(PAG)成分显示出最多的显着变化。种子到体素的FC分析(假设驱动)然后集中在PAG和NAc上,PAG和NAc被认为是关键的疼痛和奖励中心。在CTL小鼠中,这两个种子显示FC减少,FC减少了8个和22个基于Allen Brain Atlas的区域,而KO小鼠则没有。进一步的种子对种子定量显示,PAG和NAc种子具有下丘脑和杏仁核区域以及它们之间以及它们之间的FC修饰最高,从而揭示了大脑奖励和厌恶/疼痛中心的最强影响。总之,我们证明了羟考酮以MOR依赖的方式减少了大脑的交流,并建立了羟考酮的初步全脑FC签名。











































 京公网安备 11010802027423号
京公网安备 11010802027423号