Cell Discovery ( IF 13.0 ) Pub Date : 2019-07-09 , DOI: 10.1038/s41421-019-0103-0 Xingrun Zhang 1 , Ruili Cao 1 , Jinrong Niu 1 , Shumin Yang 1 , Huida Ma 1 , Shuai Zhao 1 , Haitao Li 1
|
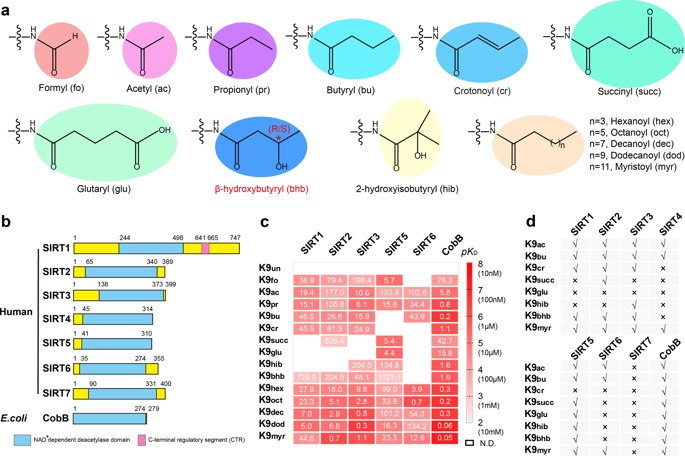
Chemical modifications on histones constitute a key mechanism for gene regulation in chromatin context. Recently, histone lysine β-hydroxybutyrylation (Kbhb) was identified as a new form of histone acylation that connects starvation-responsive metabolism to epigenetic regulation. Sirtuins are a family of NAD+-dependent deacetylases. Through systematic profiling studies, we show that human SIRT3 displays class-selective histone de-β-hydroxybutyrylase activities with preference for H3 K4, K9, K18, K23, K27, and H4K16, but not for H4 K5, K8, K12, which distinguishes it from the Zn-dependent HDACs. Structural studies revealed a hydrogen bond-lined hydrophobic pocket favored for the S-form Kbhb recognition and catalysis. β-backbone but not side chain-mediated interactions around Kbhb dominate sequence motif recognition, explaining the broad site-specificity of SIRT3. The observed class-selectivity of SIRT3 is due to an entropically unfavorable barrier associated with the glycine-flanking motif that the histone Kbhb resides in. Collectively, we reveal the molecular basis for class-selective histone de-β-hydroxybutyrylation by SIRT3, shedding lights on the function of sirtuins in Kbhb biology through hierarchical deacylation.
中文翻译:

SIRT3进行分层组蛋白去β-羟基丁酰化的分子基础。
组蛋白的化学修饰构成了染色质背景下基因调控的关键机制。最近,组蛋白赖氨酸β-羟基丁酰化(Kbhb)被确定为组蛋白酰化的一种新形式,它将饥饿反应性代谢与表观遗传调控联系起来。Sirtuins是NAD的一个家庭+依赖性脱乙酰基酶。通过系统的分析研究,我们显示人SIRT3显示类选择性组蛋白去β-羟丁酰酶活性,但优先选择H3 K4,K9,K18,K23,K27和H4K16,但不选择H4 K5,K8,K12它来自依赖锌的HDAC。结构研究表明,氢键衬里的疏水口袋有利于S型Kbhb的识别和催化。β-骨干而不是围绕Kbhb的侧链介导的相互作用主导了序列基序识别,这解释了SIRT3的广泛位点特异性。观察到的SIRT3的类选择性是由于与组蛋白Kbhb所在的甘氨酸侧翼基序相关的熵不利屏障。我们共同揭示了SIRT3的类选择性组蛋白去β-羟基丁酰化的分子基础,











































 京公网安备 11010802027423号
京公网安备 11010802027423号