Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial
The Lancet ( IF 98.4 ) Pub Date : 2019-07-04 , DOI: 10.1016/s0140-6736(19)30952-3 Kristian Reich , Melinda Gooderham , Diamant Thaçi , Jeffrey J Crowley , Caitriona Ryan , James G Krueger , Tsen-Fang Tsai , Mary Flack , Yihua Gu , David A Williams , Elizabeth H Z Thompson , Carle Paul
中文翻译:

Risankizumab与阿达木单抗在中度至重度斑块型牛皮癣(IMMvent)患者中的比较:一项随机,双盲,积极比较者对照的3期临床试验
更新日期:2019-08-16
The Lancet ( IF 98.4 ) Pub Date : 2019-07-04 , DOI: 10.1016/s0140-6736(19)30952-3 Kristian Reich , Melinda Gooderham , Diamant Thaçi , Jeffrey J Crowley , Caitriona Ryan , James G Krueger , Tsen-Fang Tsai , Mary Flack , Yihua Gu , David A Williams , Elizabeth H Z Thompson , Carle Paul
|
Background
Psoriasis is an autoimmune disease that affects approximately 100 million people worldwide, and is a disease that can be ameliorated by anti-cytokine treatment. We aimed to compare the efficacy and safety of risankizumab with adalimumab in patients with moderate-to-severe plaque psoriasis.Methods
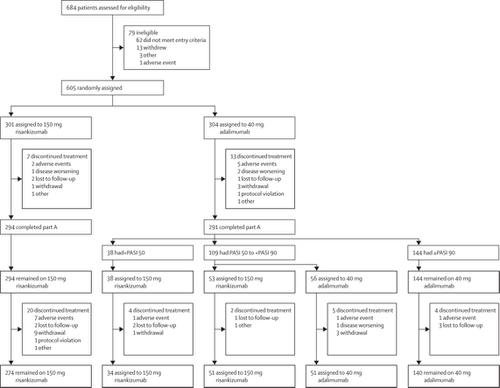
IMMvent was a phase 3, randomised, double-blind, active-comparator-controlled trial completed at 66 clinics in 11 countries. Eligible patients were aged 18 years or older with moderate-to-severe chronic plaque psoriasis. Patients were randomly assigned 1:1 using interactive response technology to receive 150 mg risankizumab subcutaneously at weeks 0 and 4 or 80 mg adalimumab subcutaneously at randomisation, then 40 mg at weeks 1, 3, 5, and every other week thereafter during a 16-week double-blind treatment period (part A). For weeks 16–44 (part B), adalimumab intermediate responders were re-randomised 1:1 to continue 40 mg adalimumab or switch to 150 mg risankizumab. In part A, participants and investigators were masked to study treatment. Randomisation was stratified by weight and previous tumour necrosis factor inhibitor exposure. Co-primary endpoints in part A were a 90% improvement from baseline (PASI 90) and a static Physician's Global Assessment (sPGA) score of 0 or 1 at week 16, and for part B was PASI 90 at week 44 (non-responder imputation). Efficacy analyses were done in the intention-to-treat population and safety analyses were done in the safety population (all patients who received at least one dose of study drug or placebo). This study is registered with , number .Findings
Between March 31, 2016, and Aug 24, 2017, 605 patients were randomly assigned to receive either risankizumab (n=301, 50%) or adalimumab (n=304, 50%). 294 (98%) of patients in the risankizumab group and 291 (96%) in the adalimumab group completed part A, and 51 (96%) of 53 patients re-randomised to risankizumab and 51 (91%) of 56 patients re-randomised to continue adalimumab completed part B. At week 16, PASI 90 was achieved in 218 (72%) of 301 patients given risankizumab and 144 (47%) of 304 patients given adalimumab (adjusted absolute difference 24·9% [95% CI 17·5–32·4]; p<0·0001), and sPGA scores of 0 or 1 were achieved in 252 (84%) patients given risankizumab and 252 (60%) patients given adalimumab (adjusted absolute difference 23·3% [16·6–30·1]; p<0·0001). In part B, among adalimumab intermediate responders, PASI 90 was achieved by 35 (66%) of 53 patients switched to risankizumab and 12 (21%) of 56 patients continuing adalimumab (adjusted absolute difference 45·0% [28·9–61·1]; p<0·0001) at week 44. Adverse events were reported in 168 (56%) of 301 patients given risankizumab and 179 (57%) of 304 patients given adalimumab in part A, and among adalimumab intermediate responders, adverse events were reported in 40 (75%) of 53 patients who switched to risankizumab and 37 (66%) of 56 patients who continued adalimumab in part B.Interpretation
Risankizumab showed significantly greater efficacy than adalimumab in providing skin clearance in patients with moderate-to-severe plaque psoriasis. No additional safety concerns were identified for patients who switched from adalimumab to risankizumab. Treatment with risankizumab provides flexibility in the long-term treatment of psoriasis.Funding
AbbVie and Boehringer Ingelheim.中文翻译:

Risankizumab与阿达木单抗在中度至重度斑块型牛皮癣(IMMvent)患者中的比较:一项随机,双盲,积极比较者对照的3期临床试验











































 京公网安备 11010802027423号
京公网安备 11010802027423号