当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Arylamides via Ritter-Type Cleavage of Solid-Supported Aryltriazenes.
ACS Combinatorial Science Pub Date : 2019-07-02 , DOI: 10.1021/acscombsci.9b00096 Nicolai A Wippert 1 , Nicole Jung 1, 2 , Stefan Bräse 1, 2
ACS Combinatorial Science Pub Date : 2019-07-02 , DOI: 10.1021/acscombsci.9b00096 Nicolai A Wippert 1 , Nicole Jung 1, 2 , Stefan Bräse 1, 2
Affiliation
|
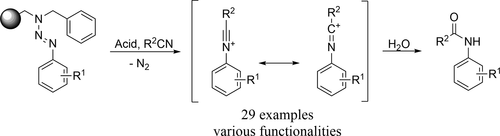
A novel route for the synthesis of N-arylamides via the cleavage of aryltriazenes with alkyl or aryl nitriles is presented. We developed a variation of the Ritter reaction that allows the use of acetonitrile as solvent and reagent in reactions with solid-supported precursors. The reaction was optimized for the generation of N-aryl acetamides using a diverse range of immobilized building blocks including o-, m-, and p-substituted aryltriazenes. The cleavage via the Ritter-type conversion was combined with an on-bead cross-coupling reaction of halogen-substituted aryltriazenes with pyrazoles. Additionally, the synthesis of on-bead generated arylboronic ester-substituted triazenes was shown. The developed procedure was further expanded to use other commercially available nitriles, such as acrylonitrile, benzonitrile, and chlorinated alkyl nitriles as suitable reagents for a Ritter-type cleavage of the prepared triazene linkers.
中文翻译:

通过固体负载的芳基三氮烯的Ritter型裂解合成芳酰胺。
提出了一种通过烷基或芳基腈裂解芳基三氮烯来合成N-芳基酰胺的新途径。我们开发了Ritter反应的一种变体,该变体允许在与固体负载前体的反应中使用乙腈作为溶剂和试剂。使用包括邻位,间位和对位取代的芳基三氮烯在内的多种固定结构单元,可以优化反应生成N-芳基乙酰胺的能力。经由Ritter型转化的裂解与卤素取代的芳基三氮烯与吡唑的珠上交叉偶联反应结合。另外,显示了珠上生成的芳基硼酸酯取代的三氮烯的合成。进一步扩展了开发的程序,以使用其他可商购的腈,例如丙烯腈,苄腈,
更新日期:2019-06-18
中文翻译:

通过固体负载的芳基三氮烯的Ritter型裂解合成芳酰胺。
提出了一种通过烷基或芳基腈裂解芳基三氮烯来合成N-芳基酰胺的新途径。我们开发了Ritter反应的一种变体,该变体允许在与固体负载前体的反应中使用乙腈作为溶剂和试剂。使用包括邻位,间位和对位取代的芳基三氮烯在内的多种固定结构单元,可以优化反应生成N-芳基乙酰胺的能力。经由Ritter型转化的裂解与卤素取代的芳基三氮烯与吡唑的珠上交叉偶联反应结合。另外,显示了珠上生成的芳基硼酸酯取代的三氮烯的合成。进一步扩展了开发的程序,以使用其他可商购的腈,例如丙烯腈,苄腈,











































 京公网安备 11010802027423号
京公网安备 11010802027423号