当前位置:
X-MOL 学术
›
Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AAV9 Vector: a Novel modality in gene therapy for spinal muscular atrophy.
Gene Therapy ( IF 4.6 ) Pub Date : 2019-06-26 , DOI: 10.1038/s41434-019-0085-4 Rithu Pattali 1 , Yongchao Mou 1, 2 , Xue-Jun Li 1, 2
Gene Therapy ( IF 4.6 ) Pub Date : 2019-06-26 , DOI: 10.1038/s41434-019-0085-4 Rithu Pattali 1 , Yongchao Mou 1, 2 , Xue-Jun Li 1, 2
Affiliation
|
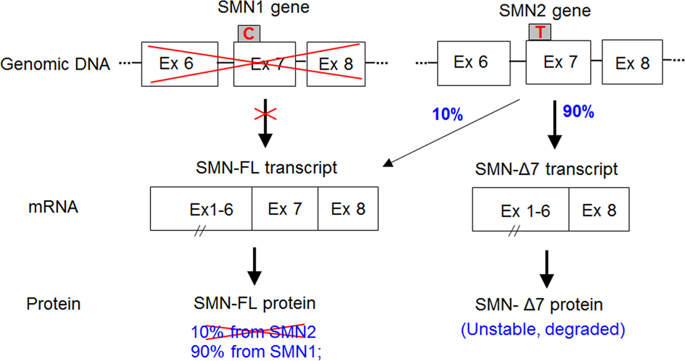
Spinal muscular atrophy (SMA), the leading genetic cause of infant mortality, is characterized by the deterioration of alpha motor neurons in the brainstem and spinal cord. Currently, there is no cure for SMA, which calls for an urgent need to explore affordable and effective therapies and to maximize patients' independence and quality of life. Adeno-associated virus (AAV) vector, one of the most promising and well-investigated vehicles for delivering transgenes, is a compelling candidate for gene therapy. Some of the hallmarks of AAVs are their nonpathogenicity, inability to incur an immune response, potential to achieve robust transgene expression, and varied tropism for several tissues of the body. Recently, these features were harnessed in a clinical trial conducted by AveXis in SMA patients, where AAV9 was employed as a vehicle for one-time administration of the SMN gene, the causative gene in SMA. The trial demonstrated remarkable improvements in motor milestones and rates of survival in the patients. This review focuses on the advent of SMA gene therapy and summarizes different preclinical studies that were conducted leading up to the AAV9-SMA trial in SMA patients.
中文翻译:

AAV9 载体:脊髓性肌萎缩症基因治疗的一种新方式。
脊髓性肌萎缩症 (SMA) 是婴儿死亡的主要遗传原因,其特征是脑干和脊髓中的 α 运动神经元退化。目前,SMA 尚无治愈方法,迫切需要探索负担得起的有效疗法,并最大限度地提高患者的独立性和生活质量。腺相关病毒 (AAV) 载体是最有前途和研究最充分的传递转基因的载体之一,是基因治疗的有力候选者。AAV 的一些标志是它们的非致病性、无法引起免疫反应、实现强大的转基因表达的潜力以及对身体的几个组织的不同趋向性。最近,AveXis 在 SMA 患者中进行的一项临床试验利用了这些特征,其中 AAV9 被用作一次性管理 SMN 基因的载体,SMN 基因是 SMA 的致病基因。该试验证明了患者运动里程碑和存活率的显着改善。本综述重点关注 SMA 基因治疗的出现,并总结了在 SMA 患者中进行 AAV9-SMA 试验之前进行的不同临床前研究。
更新日期:2019-11-18
中文翻译:

AAV9 载体:脊髓性肌萎缩症基因治疗的一种新方式。
脊髓性肌萎缩症 (SMA) 是婴儿死亡的主要遗传原因,其特征是脑干和脊髓中的 α 运动神经元退化。目前,SMA 尚无治愈方法,迫切需要探索负担得起的有效疗法,并最大限度地提高患者的独立性和生活质量。腺相关病毒 (AAV) 载体是最有前途和研究最充分的传递转基因的载体之一,是基因治疗的有力候选者。AAV 的一些标志是它们的非致病性、无法引起免疫反应、实现强大的转基因表达的潜力以及对身体的几个组织的不同趋向性。最近,AveXis 在 SMA 患者中进行的一项临床试验利用了这些特征,其中 AAV9 被用作一次性管理 SMN 基因的载体,SMN 基因是 SMA 的致病基因。该试验证明了患者运动里程碑和存活率的显着改善。本综述重点关注 SMA 基因治疗的出现,并总结了在 SMA 患者中进行 AAV9-SMA 试验之前进行的不同临床前研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号