当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elimination profile of triamcinolone hexacetonide and its metabolites in human urine and plasma after a single intra-articular administration.
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2019-06-17 , DOI: 10.1002/dta.2614 Sergi Coll 1, 2 , Xavier Matabosch 1 , Jone Llorente-Onaindia 3, 4 , Marcel Li Carbó 2 , Clara Pérez-Mañá 5, 6 , Nuria Monfort 1 , Jordi Monfort 3, 4 , Rosa Ventura 1, 2
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2019-06-17 , DOI: 10.1002/dta.2614 Sergi Coll 1, 2 , Xavier Matabosch 1 , Jone Llorente-Onaindia 3, 4 , Marcel Li Carbó 2 , Clara Pérez-Mañá 5, 6 , Nuria Monfort 1 , Jordi Monfort 3, 4 , Rosa Ventura 1, 2
Affiliation
|
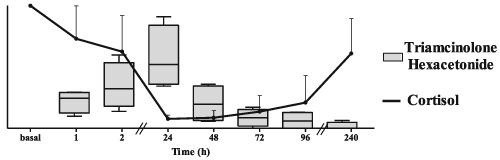
Triamcinolone hexacetonide (THA) is a synthetic glucocorticoid (GC) used by intra‐articular (IA) administration. GCs are prohibited in sports competitions by systemic routes, and they are allowed by other routes considered of local action (IA administration, among others). The aim of the present work was to study the metabolic profile of THA in urine and plasma following IA administration. Eight patients (4 males and 4 females) with knee osteoarthritis received an IA dose of THA (40 mg) in the knee joint. Spot urine and plasma samples were collected before injection and at different time periods up to day 23 and 10 post‐administration, respectively. The samples were analysed by liquid chromatography‐tandem mass spectrometry. Neither THA nor specific THA metabolites were detected in urine. Triamcinolone acetonide (TA) and 6β‐hydroxy‐triamcinolone acetonide were the main urinary metabolites. Maximum concentrations wereobtained between 24 and 48 h after administration. Using the reporting level of 30 ng/mL to distinguish allowed from forbidden administrations of GCs, a large number of false adverse analytical findings would be reported up to day 4. On the other hand, TA was detected in all plasma samples collected up to day 10 after administration. THA was also detected in plasma but at lower concentrations. The detection of plasma THA would be an unequivocal proof to demonstrate IA use of THA. A reversible decrease was observed in plasma concentrations of cortisol in some of the patients, indicating a systemic effect of the drug.
中文翻译:

单次关节内给药后,曲安西龙己烯酮及其代谢产物在人尿液和血浆中的清除曲线。
曲安奈德(THA)是一种合成的糖皮质激素(GC),用于关节内(IA)给药。在体育竞赛中,系统性路线禁止使用GC,而其他被认为是当地行动的路线(例如IA管理)也允许使用GC。本工作的目的是研究IA给药后尿液和血浆中THA的代谢情况。八名膝骨关节炎患者(男4例,女4例)在膝关节接受IA剂量的THA(40毫克)。分别在注射前和给药后第23天和第10天的不同时间点收集尿液和血浆样品。通过液相色谱-串联质谱法分析样品。尿液中均未检测到THA或特定的THA代谢产物。曲安奈德(TA)和6β-羟基曲安奈德是主要的尿代谢产物。给药后24至48小时达到最大浓度。使用30 ng / mL的报告水平来区分允许的GC和禁止的GC使用,直到第4天都会报告大量错误的不利分析结果。另一方面,直到今天,所有血浆样品中都检测到了TA。给药后10。在血浆中也检测到THA,但浓度较低。血浆THA的检测将明确证明IA使用THA。在某些患者中观察到血浆皮质醇浓度的可逆降低,表明该药物具有全身作用。给药后24至48小时达到最大浓度。使用30 ng / mL的报告水平来区分允许的GC和禁止的GC使用,直到第4天都会报告大量错误的不利分析结果。另一方面,直到今天,所有血浆样品中都检测到了TA。给药后10。在血浆中也检测到THA,但浓度较低。血浆THA的检测将明确证明IA使用THA。在某些患者中观察到血浆皮质醇浓度的可逆降低,表明该药物具有全身作用。给药后24至48小时达到最大浓度。使用30 ng / mL的报告水平来区分允许的GC和禁止的GC使用,直到第4天都会报告大量错误的不利分析结果。另一方面,直到今天,所有血浆样品中都检测到了TA。给药后10。在血浆中也检测到THA,但浓度较低。血浆THA的检测将明确证明IA使用THA。在某些患者中观察到血浆皮质醇浓度的可逆降低,表明该药物具有全身作用。另一方面,直至给药后第10天,在所有收集的血浆样品中均检测到TA。在血浆中也检测到THA,但浓度较低。血浆THA的检测将明确证明IA使用THA。在某些患者中观察到血浆皮质醇浓度的可逆降低,表明该药物具有全身作用。另一方面,直至给药后第10天,在所有收集的血浆样品中均检测到TA。在血浆中也检测到THA,但浓度较低。血浆THA的检测将明确证明IA使用THA。在某些患者中观察到血浆皮质醇浓度的可逆降低,表明该药物具有全身作用。
更新日期:2019-06-17
中文翻译:

单次关节内给药后,曲安西龙己烯酮及其代谢产物在人尿液和血浆中的清除曲线。
曲安奈德(THA)是一种合成的糖皮质激素(GC),用于关节内(IA)给药。在体育竞赛中,系统性路线禁止使用GC,而其他被认为是当地行动的路线(例如IA管理)也允许使用GC。本工作的目的是研究IA给药后尿液和血浆中THA的代谢情况。八名膝骨关节炎患者(男4例,女4例)在膝关节接受IA剂量的THA(40毫克)。分别在注射前和给药后第23天和第10天的不同时间点收集尿液和血浆样品。通过液相色谱-串联质谱法分析样品。尿液中均未检测到THA或特定的THA代谢产物。曲安奈德(TA)和6β-羟基曲安奈德是主要的尿代谢产物。给药后24至48小时达到最大浓度。使用30 ng / mL的报告水平来区分允许的GC和禁止的GC使用,直到第4天都会报告大量错误的不利分析结果。另一方面,直到今天,所有血浆样品中都检测到了TA。给药后10。在血浆中也检测到THA,但浓度较低。血浆THA的检测将明确证明IA使用THA。在某些患者中观察到血浆皮质醇浓度的可逆降低,表明该药物具有全身作用。给药后24至48小时达到最大浓度。使用30 ng / mL的报告水平来区分允许的GC和禁止的GC使用,直到第4天都会报告大量错误的不利分析结果。另一方面,直到今天,所有血浆样品中都检测到了TA。给药后10。在血浆中也检测到THA,但浓度较低。血浆THA的检测将明确证明IA使用THA。在某些患者中观察到血浆皮质醇浓度的可逆降低,表明该药物具有全身作用。给药后24至48小时达到最大浓度。使用30 ng / mL的报告水平来区分允许的GC和禁止的GC使用,直到第4天都会报告大量错误的不利分析结果。另一方面,直到今天,所有血浆样品中都检测到了TA。给药后10。在血浆中也检测到THA,但浓度较低。血浆THA的检测将明确证明IA使用THA。在某些患者中观察到血浆皮质醇浓度的可逆降低,表明该药物具有全身作用。另一方面,直至给药后第10天,在所有收集的血浆样品中均检测到TA。在血浆中也检测到THA,但浓度较低。血浆THA的检测将明确证明IA使用THA。在某些患者中观察到血浆皮质醇浓度的可逆降低,表明该药物具有全身作用。另一方面,直至给药后第10天,在所有收集的血浆样品中均检测到TA。在血浆中也检测到THA,但浓度较低。血浆THA的检测将明确证明IA使用THA。在某些患者中观察到血浆皮质醇浓度的可逆降低,表明该药物具有全身作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号