Oncogenesis ( IF 5.9 ) Pub Date : 2019-06-17 , DOI: 10.1038/s41389-019-0147-x Anne M van Harten 1 , Marijke Buijze 1 , Richard van der Mast 1 , Martin A Rooimans 2 , Sanne R Martens-de Kemp 1 , Costa Bachas 1 , Arjen Brink 1 , Marijke Stigter-van Walsum 1 , Rob M F Wolthuis 2 , Ruud H Brakenhoff 1
|
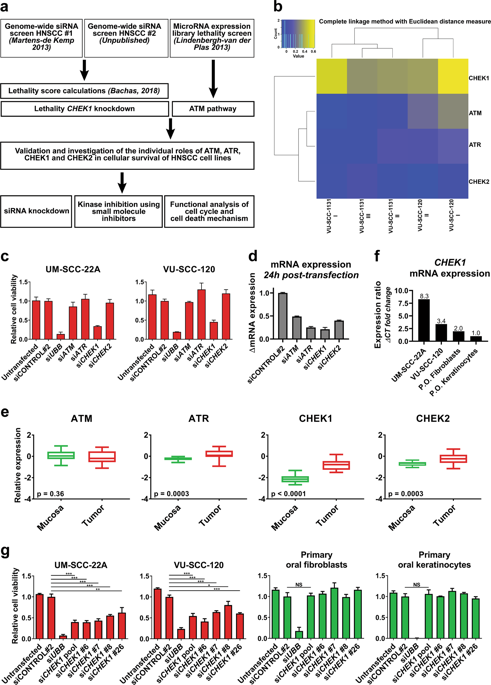
Head and neck squamous cell carcinomas (HNSCCs) coincide with poor survival rates. The lack of driver oncogenes complicates the development of targeted treatments for HNSCC. Here, we follow-up on two previous genome-wide RNA and microRNA interference screens in HNSCC to cross-examine tumor-specific lethality by targeting ATM, ATR, CHEK1, or CHEK2. Our results uncover CHEK1 as the most promising target for HNSCC. CHEK1 expression is essential across a panel of HNSCC cell lines but redundant for growth and survival of untransformed oral keratinocytes and fibroblasts. LY2603618 (Rabusertib), which specifically targets Chk1 kinase, kills HNSCC cells effectively and specifically. Our findings show that HNSCC cells depend on Chk1-mediated signaling to progress through S-phase successfully. Chk1 inhibition coincides with stalled DNA replication, replication fork collapses, and accumulation of DNA damage. We further show that Chk1 inhibition leads to bimodal HNSCC cell killing. In the most sensitive cell lines, apoptosis is induced in S-phase, whereas more resistant cell lines manage to bypass replication-associated apoptosis, but accumulate chromosomal breaks that become lethal in subsequent mitosis. Interestingly, CDK1 expression correlates with treatment outcome. Moreover, sensitivity to Chk1 inhibition requires functional CDK1 and CDK4/6 to drive cell cycle progression, arguing against combining Chk1 inhibitors with CDK inhibitors. In contrast, Wee1 inhibitor Adavosertib progresses the cell cycle and thereby increases lethality to Chk1 inhibition in HNSCC cell lines. We conclude that Chk1 has become a key molecule in HNSCC cell cycle regulation and a very promising therapeutic target. Chk1 inhibition leads to S-phase apoptosis or death in mitosis. We provide a potential efficacy biomarker and combination therapy to follow-up in clinical setting.
中文翻译:

通过 Chk1 抑制靶向头颈癌的细胞周期:双模式细胞死亡的新概念。
头颈鳞状细胞癌 (HNSCC) 的生存率较低。驱动癌基因的缺乏使得 HNSCC 靶向治疗的开发变得复杂。在这里,我们对之前在 HNSCC 中进行的两次全基因组 RNA 和 microRNA 干扰筛选进行了跟踪,通过靶向ATM 、 ATR 、 CHEK1或CHEK2来交叉检查肿瘤特异性致死性。我们的结果表明CHEK1是 HNSCC 最有希望的靶点。 CHEK1表达对于一组 HNSCC 细胞系至关重要,但对于未转化的口腔角质形成细胞和成纤维细胞的生长和存活来说是多余的。 LY2603618 (Rabusertib) 专门针对 Chk1 激酶,有效且特异性地杀死 HNSCC 细胞。我们的研究结果表明,HNSCC 细胞依赖 Chk1 介导的信号传导成功地进入 S 期。 Chk1 抑制与 DNA 复制停滞、复制叉崩溃和 DNA 损伤累积同时发生。我们进一步表明 Chk1 抑制导致双峰 HNSCC 细胞杀伤。在最敏感的细胞系中,细胞凋亡在 S 期被诱导,而更具抵抗力的细胞系设法绕过复制相关的细胞凋亡,但会积累染色体断裂,在随后的有丝分裂中变得致命。有趣的是,CDK1 表达与治疗结果相关。此外,对 Chk1 抑制的敏感性需要功能性 CDK1 和 CDK4/6 来驱动细胞周期进展,因此反对将 Chk1 抑制剂与 CDK 抑制剂联合使用。相比之下,Wee1 抑制剂 Adavosertib 会促进细胞周期,从而增加 HNSCC 细胞系中 Chk1 抑制的致死率。我们得出结论,Chk1 已成为 HNSCC 细胞周期调节的关键分子和非常有前途的治疗靶点。 Chk1 抑制导致 S 期细胞凋亡或有丝分裂死亡。我们提供潜在疗效的生物标志物和联合疗法以进行临床随访。









































 京公网安备 11010802027423号
京公网安备 11010802027423号