NPG Asia Materials ( IF 8.6 ) Pub Date : 2019-06-14 , DOI: 10.1038/s41427-019-0128-8 Marta Tunesi , Ilaria Raimondi , Teresa Russo , Laura Colombo , Edoardo Micotti , Edoardo Brandi , Pamela Cappelletti , Alberto Cigada , Alessandro Negro , Luigi Ambrosio , Gianluigi Forloni , Loredano Pollegioni , Antonio Gloria , Carmen Giordano , Diego Albani
|
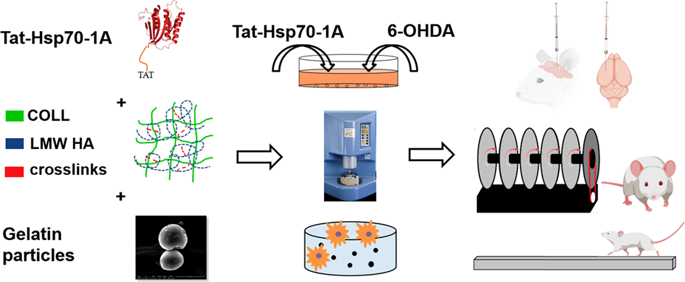
Neurodegenerative disorders such as Parkinson’s disease (PD) have no effective therapies. However, many promising drugs are precluded from clinical trials because of their poor brain availability. The chaperone protein Hsp70 has been reported to be effective in PD models, but its brain targeting is challenging. We developed a novel brain Hsp70 delivery system using injectable, biocompatible, and biodegradable semi-interpenetrating polymer networks of collagen (COLL) and low-molecular-weight hyaluronic acid (LMW HA) structured with gelatin particles. We produced human recombinant Hsp70-1A fused with the cell-penetrating peptide Tat (Tat-Hsp70) that was neuroprotective in vitro against the dopaminergic toxin 6-hydroxydopamine (6-OHDA). We assessed Tat-Hsp70 release from the selected COLL-LMW HA composites in vitro, observing a 95% release of loaded protein after 96 h. The release kinetics FITTED the Korsmeyer-Peppas model (regression coefficient 0.98) and the released Tat-Hsp70 remained neuroprotective for SH-SY5Y cells. Magnetic resonance imaging revealed that COLL-LMW HA composites lasted at least 96 h at the brain level, and in vivo Tat-Hsp70 release studies indicated that hydrogel presence is pivotal for a spatially focused neuroprotective effect. In an in vivo model of dopaminergic degeneration, Tat-Hsp70-loaded composites conveyed neuroprotection at both the behavioral and dopaminergic neuronal levels against the striatal injection of 6-OHDA. After the injection of Tat-Hsp70-loaded composites, mice showed a transient inflammatory response, with a decrease in GFAP and CD11b immunostaining after 7 days. Our delivery system enabled the effective brain release of Tat-Hsp70 and is ready for further improvements.
中文翻译:

基于水凝胶的Tat融合蛋白Hsp70的递送可在体外和帕金森氏病小鼠模型中保护多巴胺能细胞
诸如帕金森氏病(PD)的神经退行性疾病没有有效的疗法。但是,由于大脑的可利用性差,许多有前途的药物被排除在临床试验之外。据报道,伴侣蛋白Hsp70在PD模型中是有效的,但是其脑靶向性具有挑战性。我们开发了一种新型的大脑Hsp70输送系统,该系统使用了具有明胶颗粒的胶原蛋白(COLL)和低分子量透明质酸(LMW HA)的可注射,生物相容性和可生物降解的半互穿聚合物网络。我们生产了与细胞穿透肽Tat(Tat-Hsp70)融合的人重组Hsp70-1A,该肽在体外对多巴胺能毒素6-羟基多巴胺(6-OHDA)具有神经保护作用。我们评估了所选COLL-LMW HA复合材料在体外的Tat-Hsp70释放,在96小时后观察到95%的负载蛋白释放。释放动力学符合Korsmeyer-Peppas模型(回归系数0.98),释放的Tat-Hsp70对SH-SY5Y细胞仍然具有神经保护作用。磁共振成像显示COLL-LMW HA复合材料在大脑水平持续至少96小时,体内Tat-Hsp70释放研究表明水凝胶的存在对于空间聚焦神经保护作用至关重要。在多巴胺能变性的体内模型中,装有Tat-Hsp70的复合材料在行为和多巴胺能神经元水平上均能抵御纹状体注射6-OHDA的神经保护作用。注射载有Tat-Hsp70的复合物后,小鼠表现出短暂的炎症反应,7天后GFAP和CD11b免疫染色降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号