当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer.
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.34953 Chao Hong 1 , Dan Wang 1, 2 , Jianming Liang 1, 3 , Yizhen Guo 1 , Ying Zhu 4 , Jiaxuan Xia 1 , Jing Qin 1 , Huaxing Zhan 2 , Jianxin Wang 1, 5
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.34953 Chao Hong 1 , Dan Wang 1, 2 , Jianming Liang 1, 3 , Yizhen Guo 1 , Ying Zhu 4 , Jiaxuan Xia 1 , Jing Qin 1 , Huaxing Zhan 2 , Jianxin Wang 1, 5
Affiliation
|
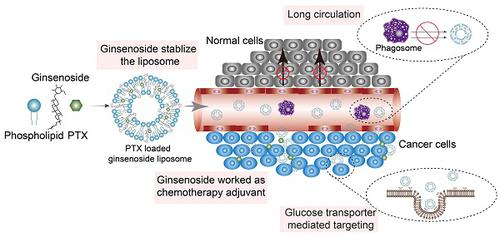
The clinical treatment of gastric cancer (GC) is hampered by the development of anticancer drug resistance and the unfavorable pharmacokinetics, off-target toxicity, and inadequate intratumoral accumulation of the current chemotherapy treatments. Ginsenosides combined with paclitaxel (PTX) have been shown to exert synergistic inhibition of human GC cell proliferation. In the present study, we developed a novel multifunctional liposome system, in which ginsenosides functioned as the chemotherapy adjuvant and membrane stabilizer. These had long blood circulation times and active targeting abilities, thus creating multifunctionality of the liposomes and facilitating drug administration to the GC cells. Methods: Three ginsenosides with different structures were used to formulate the unique nanocarrier, which was prepared using the thin-film hydration method. The stability of the ginsenoside liposomes was determined by particle size analysis using dynamic light scattering. The long circulation time of ginsenoside liposomes was compared with that of conventional liposome and polyethylene glycosylated liposomes in vivo. The active targeting effect of ginsenoside liposomes was examined with a GC xenograft model using an in vivo imaging system. To examine the antitumor activity of ginsenoside liposomes against GC, MTT, cell cycle, and apoptosis assays were performed on BGC-823 cells in vitro and PTX-loaded ginsenoside liposomes were prepared to evaluate the therapeutic efficacy on GC in vivo. Results: The ginsenosides stabilized the liposomes in a manner similar to cholesterol. We confirmed the successful delivery of the bioactive combination drugs and internalization into GC cells via analysis of the glucose-related transporter recognition and longer blood circulation time. PTX was encapsulated in different liposomal formulations for use as a combination therapy, in which ginsenosides were found to exert their inherent anticancer activity, as well as act synergistically with PTX. The combination therapy using these targeted liposomes significantly suppressed GC tumor growth and outperformed most reported PTX formulations, including Lipusu® and Abraxane®. Conclusion: We established novel ginsenoside-based liposomes as a tumor-targeting therapy, in which ginsenoside functioned not only as a chemotherapy adjuvant, but also as a functional membrane material. Ginsenoside-based liposomes offer a novel platform for anticancer drug delivery and may lead to a new era of nanocarrier treatments for cancer.
中文翻译:

基于人参皂苷的新型多功能脂质体递送系统,用于胃癌的联合治疗。
胃癌(GC)的临床治疗受到抗癌药耐药性的发展以及目前化学疗法不利的药代动力学,脱靶毒性和肿瘤内累积不足的困扰。人参皂苷与紫杉醇(PTX)的结合已显示出对人类GC细胞增殖的协同抑制作用。在本研究中,我们开发了一种新型的多功能脂质体系统,其中人参皂甙起着化疗辅助剂和膜稳定剂的作用。它们具有长的血液循环时间和有效的靶向能力,因此产生脂质体的多功能性并促进药物向GC细胞的给药。方法:使用三种不同结构的人参皂苷来配制独特的纳米载体,用薄膜水化法制备。人参皂苷脂质体的稳定性通过使用动态光散射的粒度分析来确定。将人参皂苷脂质体的长循环时间与常规脂质体和聚乙烯糖基化脂质体的体内循环时间进行了比较。使用体内成像系统,采用GC异种移植模型检查了人参皂苷脂质体的主动靶向作用。为了检查人参皂苷脂质体对GC的抗肿瘤活性,在体外对BGC-823细胞进行了测定,并制备了负载PTX的人参皂苷脂质体以评估其对GC的治疗效果。结果:人参皂甙以类似于胆固醇的方式稳定了脂质体。我们通过对葡萄糖相关转运蛋白的识别和更长的血液循环时间进行分析,确认了生物活性组合药物的成功递送和内在化进入GC细胞。PTX被封装在不同的脂质体制剂中,以用作组合疗法,其中人参皂甙被发现发挥其固有的抗癌活性,并与PTX协同发挥作用。使用这些靶向脂质体的联合疗法显着抑制了GC肿瘤的生长,并且优于大多数报道的PTX制剂,包括Lipusu®和Abraxane®。结论:我们建立了新的基于人参皂苷的脂质体作为肿瘤靶向疗法,其中人参皂苷不仅起着化疗佐剂的作用,而且还起着功能性膜材料的作用。
更新日期:2019-01-01
中文翻译:

基于人参皂苷的新型多功能脂质体递送系统,用于胃癌的联合治疗。
胃癌(GC)的临床治疗受到抗癌药耐药性的发展以及目前化学疗法不利的药代动力学,脱靶毒性和肿瘤内累积不足的困扰。人参皂苷与紫杉醇(PTX)的结合已显示出对人类GC细胞增殖的协同抑制作用。在本研究中,我们开发了一种新型的多功能脂质体系统,其中人参皂甙起着化疗辅助剂和膜稳定剂的作用。它们具有长的血液循环时间和有效的靶向能力,因此产生脂质体的多功能性并促进药物向GC细胞的给药。方法:使用三种不同结构的人参皂苷来配制独特的纳米载体,用薄膜水化法制备。人参皂苷脂质体的稳定性通过使用动态光散射的粒度分析来确定。将人参皂苷脂质体的长循环时间与常规脂质体和聚乙烯糖基化脂质体的体内循环时间进行了比较。使用体内成像系统,采用GC异种移植模型检查了人参皂苷脂质体的主动靶向作用。为了检查人参皂苷脂质体对GC的抗肿瘤活性,在体外对BGC-823细胞进行了测定,并制备了负载PTX的人参皂苷脂质体以评估其对GC的治疗效果。结果:人参皂甙以类似于胆固醇的方式稳定了脂质体。我们通过对葡萄糖相关转运蛋白的识别和更长的血液循环时间进行分析,确认了生物活性组合药物的成功递送和内在化进入GC细胞。PTX被封装在不同的脂质体制剂中,以用作组合疗法,其中人参皂甙被发现发挥其固有的抗癌活性,并与PTX协同发挥作用。使用这些靶向脂质体的联合疗法显着抑制了GC肿瘤的生长,并且优于大多数报道的PTX制剂,包括Lipusu®和Abraxane®。结论:我们建立了新的基于人参皂苷的脂质体作为肿瘤靶向疗法,其中人参皂苷不仅起着化疗佐剂的作用,而且还起着功能性膜材料的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号