当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ZnAs@SiO2 nanoparticles as a potential anti-tumor drug for targeting stemness and epithelial-mesenchymal transition in hepatocellular carcinoma via SHP-1/JAK2/STAT3 signaling.
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.32462 Yongquan Huang 1, 2, 3 , Bin Zhou 2, 3 , Hui Luo 3 , Junjie Mao 2, 3 , Yin Huang 2 , Ke Zhang 2, 3 , Chaoming Mei 3 , Yan Yan 3 , Hongjun Jin 3 , Jinhao Gao 4 , Zhongzhen Su 1, 3 , Pengfei Pang 2, 3 , Dan Li 3 , Hong Shan 2, 3
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.32462 Yongquan Huang 1, 2, 3 , Bin Zhou 2, 3 , Hui Luo 3 , Junjie Mao 2, 3 , Yin Huang 2 , Ke Zhang 2, 3 , Chaoming Mei 3 , Yan Yan 3 , Hongjun Jin 3 , Jinhao Gao 4 , Zhongzhen Su 1, 3 , Pengfei Pang 2, 3 , Dan Li 3 , Hong Shan 2, 3
Affiliation
|
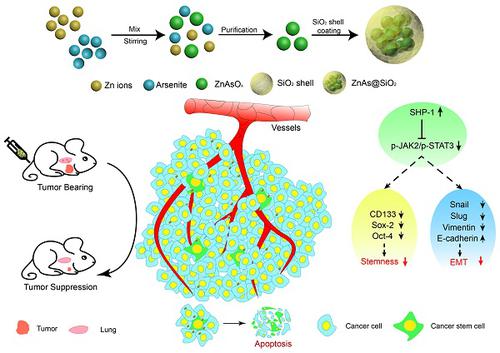
Rationale: Current therapies for hepatocellular carcinoma (HCC) are hampered by treatment failure and recurrence due to the remaining treatment-resistant liver cancer stem cells (CSCs). Stemness and epithelial-mesenchymal transition (EMT) are regarded as two fundamental characteristics of liver CSCs necessary for cancer progression; thus, drugs that simultaneously target both characteristics should prove effective in eliminating HCC and impeding recurrence. In this study, we developed new arsenic trioxide (ATO)-based nanoparticles (NPs), which are expected to be more effective than the current HCC therapy, and explored their potential mechanism. Methods: A "one-pot" reverse emulsification approach was employed to prepare the ZnAs@SiO2 NPs. HCC cell lines, MHCC97L and Hep3b, were used to analyze the antitumor activity of ZnAs@SiO2 NPs in vitro and in vivo by quantifying cell growth and metastasis as well as to study the effect on stemness and EMT. SHP-1 siRNA was used to validate the role of the SHP-1/JAK2/STAT3 signaling pathway in mediating inhibition of stemness and EMT by ZnAs@SiO2. Results: Compared with the current ATO treatment, ZnAs@SiO2 NPs promoted apoptosis and significantly inhibited proliferation, migration, and invasion of both MHCC97L and Hep3b cells. In the in vivo assay, ZnAs@SiO2 NPs inhibited tumor growth by 2.2-fold and metastasis by 3.5-fold as compared to ATO. The ZnAs@SiO2 NPs also inhibited tumor spheroid formation in vitro and tumor initiation in vivo and induced significant changes in the expression of stemness markers (CD133, Sox-2, and Oct-4) and EMT markers (E-cadherin, Vimentin, and Slug) both in vitro and in vivo. These effects of ZnAs@SiO2 that correlated with prognosis of HCC were mediated by the SHP-1/JAK2/STAT3 signaling. Conclusions: ZnAs@SiO2 NPs can effectively suppress tumor initiation, growth, metastasis, and inhibit stemness and EMT through regulation of SHP-1/JAK2/STAT3 signaling pathway in liver cancer cells in vitro and in vivo. Thus, ZnAs@SiO2 NPs have immense potential for HCC treatment in the future.
中文翻译:

ZnAs @ SiO2纳米颗粒可作为潜在的抗肿瘤药物,通过SHP-1 / JAK2 / STAT3信号传导靶向肝细胞癌的干性和上皮-间质转化。
理由:由于剩余的抗治疗性肝癌干细胞(CSC),治疗失败和复发阻碍了肝细胞癌(HCC)的当前疗法。茎干和上皮-间质转化(EMT)被认为是癌症进展所必需的肝CSC的两个基本特征。因此,同时针对这两种特性的药物应能有效消除HCC并阻止复发。在这项研究中,我们开发了新的基于三氧化二砷(ATO)的纳米颗粒(NPs),并有望比目前的HCC治疗更有效,并探讨了其潜在机理。方法:采用“一锅法”逆乳化法制备ZnAs @ SiO2 NPs。HCC细胞系MHCC97L和Hep3b,通过定量细胞生长和转移来研究ZnAs @ SiO2 NPs的体内外抗肿瘤活性,以及研究其对干细胞和EMT的影响。SHP-1 siRNA用于验证SHP-1 / JAK2 / STAT3信号通路在介导ZnAs @ SiO2抑制干性和EMT方面的作用。结果:与目前的ATO处理相比,ZnAs @ SiO2 NPs促进了MHCC97L和Hep3b细胞的凋亡并显着抑制了它们的增殖,迁移和侵袭。在体内试验中,与ATO相比,ZnAs @ SiO2 NPs抑制肿瘤生长2.2倍,转移抑制3.5倍。ZnAs @ SiO2 NPs还可以在体外和体内抑制肿瘤球体的形成,并诱导干性标记(CD133,Sox-2和Oct-4)和EMT标记(E-钙粘着蛋白,波形蛋白,和Slug)。SHP-1 / JAK2 / STAT3信号介导了ZnAs @ SiO2与HCC预后相关的这些作用。结论:ZnAs @ SiO2 NPs通过调节SHP-1 / JAK2 / STAT3信号通路有效抑制肝癌细胞的肿瘤发生,生长,转移,并抑制干细胞和EMT。因此,ZnAs @ SiO2 NPs在未来的HCC处理中具有巨大的潜力。
更新日期:2019-01-01
中文翻译:

ZnAs @ SiO2纳米颗粒可作为潜在的抗肿瘤药物,通过SHP-1 / JAK2 / STAT3信号传导靶向肝细胞癌的干性和上皮-间质转化。
理由:由于剩余的抗治疗性肝癌干细胞(CSC),治疗失败和复发阻碍了肝细胞癌(HCC)的当前疗法。茎干和上皮-间质转化(EMT)被认为是癌症进展所必需的肝CSC的两个基本特征。因此,同时针对这两种特性的药物应能有效消除HCC并阻止复发。在这项研究中,我们开发了新的基于三氧化二砷(ATO)的纳米颗粒(NPs),并有望比目前的HCC治疗更有效,并探讨了其潜在机理。方法:采用“一锅法”逆乳化法制备ZnAs @ SiO2 NPs。HCC细胞系MHCC97L和Hep3b,通过定量细胞生长和转移来研究ZnAs @ SiO2 NPs的体内外抗肿瘤活性,以及研究其对干细胞和EMT的影响。SHP-1 siRNA用于验证SHP-1 / JAK2 / STAT3信号通路在介导ZnAs @ SiO2抑制干性和EMT方面的作用。结果:与目前的ATO处理相比,ZnAs @ SiO2 NPs促进了MHCC97L和Hep3b细胞的凋亡并显着抑制了它们的增殖,迁移和侵袭。在体内试验中,与ATO相比,ZnAs @ SiO2 NPs抑制肿瘤生长2.2倍,转移抑制3.5倍。ZnAs @ SiO2 NPs还可以在体外和体内抑制肿瘤球体的形成,并诱导干性标记(CD133,Sox-2和Oct-4)和EMT标记(E-钙粘着蛋白,波形蛋白,和Slug)。SHP-1 / JAK2 / STAT3信号介导了ZnAs @ SiO2与HCC预后相关的这些作用。结论:ZnAs @ SiO2 NPs通过调节SHP-1 / JAK2 / STAT3信号通路有效抑制肝癌细胞的肿瘤发生,生长,转移,并抑制干细胞和EMT。因此,ZnAs @ SiO2 NPs在未来的HCC处理中具有巨大的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号