当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Autophagy impairment contributes to PBDE-47-induced developmental neurotoxicity and its relationship with apoptosis.
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.33688 Pei Li 1, 2 , Rulin Ma 1, 2 , Lixin Dong 1, 2 , Luming Liu 1, 2 , Guoyu Zhou 1, 2 , Zhiyuan Tian 1, 2 , Qian Zhao 1, 2 , Tao Xia 1, 2 , Shun Zhang 1, 2 , Aiguo Wang 1, 2
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.33688 Pei Li 1, 2 , Rulin Ma 1, 2 , Lixin Dong 1, 2 , Luming Liu 1, 2 , Guoyu Zhou 1, 2 , Zhiyuan Tian 1, 2 , Qian Zhao 1, 2 , Tao Xia 1, 2 , Shun Zhang 1, 2 , Aiguo Wang 1, 2
Affiliation
|
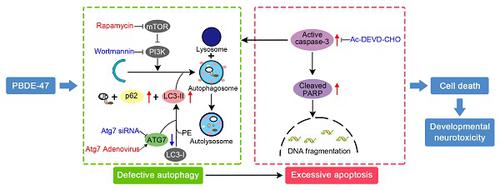
Apoptosis is involved in 2,2',4,4'- tetrabromodiphenyl ether (PBDE-47)-induced developmental neurotoxicity. However, little is known about the role of autophagy, especially its relationship with apoptosis underlying such neurotoxic process. Methods: Using female Sprague-Dawley rats exposed to low-dose PBDE-47 (0.1, 1.0 and 10 mg/kg/day) from pre-pregnancy until weaning of offspring to mimic human exposure, we investigated the effects of PBDE-47 on autophagy and apoptosis in relation to cognitive impairment of adult offspring rats. We also evaluated relationship between autophagy and apoptosis using neuroendocrine pheochromocytoma (PC12) cells, a widely used neuron-like cell line for neuronal development. Results: In vivo, perinatal exposure to PBDE-47 induced memory deficits in adult rats. This is accompanied by hippocampal neuronal loss partly as a result of apoptosis, as evidenced by caspase-3 activation and PARP cleavage. Further study identified that PBDE-47 triggered autophagic vesicles accumulation, increased levels of microtubule-associated protein 1 light chain 3 (LC3)-II, an essential protein for autophagosomes formation, and autophagy substrate sequestosome 1 (SQSTM1/p62), but reduced levels of autophagy-related protein (ATG) 7, a key protein for autophagosomes elongation, suggestive of autophagy impairment. These findings were further demonstrated by an in vitro model of PBDE-47-treated PC12 cells. Mechanistically, autophagy alteration is more sensitive to PBDE-47 treatment than apoptosis induction. Importantly, while stimulation of autophagy by the chemical inducer rapamycin and adenovirus-mediated Atg7 overexpression aggravated PBDE-47-induced apoptosis and cell death, inhibition of autophagy by the chemical inhibitor wortmannin and siRNA knockdown of Atg7 reversed PBDE-47-produced detrimental outcomes. Interestingly, blockage of apoptosis by caspase-3 inhibitor Ac-DEVD-CHO ameliorated PBDE-47-exerted autophagy impairment and cell death, though in combination with autophagy inhibitor did not further promote cell survival. Conclusion: Our data suggest that autophagy impairment facilitates apoptosis, which, in turn, disrupts autophagy, ultimately resulting in cell death, and that autophagy may act as a promising therapeutic target for PBDE-47-induced developmental neurotoxicity.
中文翻译:

自噬损伤导致PBDE-47诱导的发育神经毒性及其与凋亡的关系。
凋亡参与2,2',4,4'-四溴二苯醚(PBDE-47)诱导的发育神经毒性。然而,关于自噬的作用,尤其是其与这种神经毒性过程背后的细胞凋亡的关系,知之甚少。方法:使用从怀孕前到断奶后代模仿人类暴露的低剂量PBDE-47(0.1、1.0和10 mg / kg /天)暴露的雌性Sprague-Dawley大鼠,我们研究了PBDE-47的作用与成年后代大鼠认知障碍相关的自噬和细胞凋亡 我们还使用神经内分泌嗜铬细胞瘤(PC12)细胞(一种广泛用于神经元发育的神经元样细胞系)评估了自噬与凋亡之间的关系。结果:在体内,围产期暴露于PBDE-47导致成年大鼠记忆力减退。这伴随着海马神经元的丧失,部分是由于细胞凋亡引起的,如caspase-3激活和PARP裂解所证明的。进一步的研究发现,PBDE-47会触发自噬小泡的积累,微管相关蛋白1轻链3(LC3)-II(自噬体形成的必需蛋白)和自噬底物螯合体1(SQSTM1 / p62)的水平升高,但水平降低自噬相关蛋白(ATG)7的表达,它是自噬体延长的关键蛋白,提示自噬受损。PBDE-47处理的PC12细胞的体外模型进一步证明了这些发现。从机制上讲,自噬改变对PBDE-47的治疗比对细胞凋亡的诱导更敏感。重要的,虽然化学诱导剂雷帕霉素和腺病毒介导的Atg7过表达刺激自噬会加剧PBDE-47诱导的细胞凋亡和细胞死亡,而化学抑制剂渥曼青霉素的抑制自噬作用和Atg7的siRNA抑制作用逆转了PBDE-47产生的有害结果。有趣的是,尽管与自噬抑制剂联合使用并不能进一步促进细胞存活,但是胱天蛋白酶3抑制剂Ac-DEVD-CHO对细胞凋亡的阻断改善了PBDE-47所致的自噬损伤和细胞死亡。结论:我们的数据表明自噬损伤促进细胞凋亡,继而破坏自噬,最终导致细胞死亡,并且自噬可能成为PBDE-47诱导的发育性神经毒性的有希望的治疗靶点。化学抑制剂渥曼青霉素对自噬的抑制和Atg7的siRNA敲低逆转了PBDE-47产生的有害结果。有趣的是,尽管与自噬抑制剂联合使用并不能进一步促进细胞存活,但是胱天蛋白酶3抑制剂Ac-DEVD-CHO对细胞凋亡的阻断改善了PBDE-47所致的自噬损伤和细胞死亡。结论:我们的数据表明自噬损伤促进细胞凋亡,继而破坏自噬,最终导致细胞死亡,并且自噬可能成为PBDE-47诱导的发育性神经毒性的有希望的治疗靶点。化学抑制剂渥曼青霉素对自噬的抑制和Atg7的siRNA敲低逆转了PBDE-47产生的有害结果。有趣的是,尽管与自噬抑制剂联合使用并不能进一步促进细胞存活,但是胱天蛋白酶3抑制剂Ac-DEVD-CHO对细胞凋亡的阻断改善了PBDE-47所致的自噬损伤和细胞死亡。结论:我们的数据表明自噬损伤促进细胞凋亡,继而破坏自噬,最终导致细胞死亡,并且自噬可能成为PBDE-47诱导的发育性神经毒性的有希望的治疗靶点。caspase-3抑制剂Ac-DEVD-CHO阻滞凋亡可改善PBDE-47引起的自噬损伤和细胞死亡,尽管与自噬抑制剂联合使用不会进一步促进细胞存活。结论:我们的数据表明自噬损伤促进细胞凋亡,继而破坏自噬,最终导致细胞死亡,并且自噬可能成为PBDE-47诱导的发育性神经毒性的有希望的治疗靶点。caspase-3抑制剂Ac-DEVD-CHO阻滞凋亡可改善PBDE-47引起的自噬损伤和细胞死亡,尽管与自噬抑制剂联合使用不会进一步促进细胞存活。结论:我们的数据表明自噬损伤促进细胞凋亡,继而破坏自噬,最终导致细胞死亡,并且自噬可能成为PBDE-47诱导的发育性神经毒性的有希望的治疗靶点。
更新日期:2019-01-01
中文翻译:

自噬损伤导致PBDE-47诱导的发育神经毒性及其与凋亡的关系。
凋亡参与2,2',4,4'-四溴二苯醚(PBDE-47)诱导的发育神经毒性。然而,关于自噬的作用,尤其是其与这种神经毒性过程背后的细胞凋亡的关系,知之甚少。方法:使用从怀孕前到断奶后代模仿人类暴露的低剂量PBDE-47(0.1、1.0和10 mg / kg /天)暴露的雌性Sprague-Dawley大鼠,我们研究了PBDE-47的作用与成年后代大鼠认知障碍相关的自噬和细胞凋亡 我们还使用神经内分泌嗜铬细胞瘤(PC12)细胞(一种广泛用于神经元发育的神经元样细胞系)评估了自噬与凋亡之间的关系。结果:在体内,围产期暴露于PBDE-47导致成年大鼠记忆力减退。这伴随着海马神经元的丧失,部分是由于细胞凋亡引起的,如caspase-3激活和PARP裂解所证明的。进一步的研究发现,PBDE-47会触发自噬小泡的积累,微管相关蛋白1轻链3(LC3)-II(自噬体形成的必需蛋白)和自噬底物螯合体1(SQSTM1 / p62)的水平升高,但水平降低自噬相关蛋白(ATG)7的表达,它是自噬体延长的关键蛋白,提示自噬受损。PBDE-47处理的PC12细胞的体外模型进一步证明了这些发现。从机制上讲,自噬改变对PBDE-47的治疗比对细胞凋亡的诱导更敏感。重要的,虽然化学诱导剂雷帕霉素和腺病毒介导的Atg7过表达刺激自噬会加剧PBDE-47诱导的细胞凋亡和细胞死亡,而化学抑制剂渥曼青霉素的抑制自噬作用和Atg7的siRNA抑制作用逆转了PBDE-47产生的有害结果。有趣的是,尽管与自噬抑制剂联合使用并不能进一步促进细胞存活,但是胱天蛋白酶3抑制剂Ac-DEVD-CHO对细胞凋亡的阻断改善了PBDE-47所致的自噬损伤和细胞死亡。结论:我们的数据表明自噬损伤促进细胞凋亡,继而破坏自噬,最终导致细胞死亡,并且自噬可能成为PBDE-47诱导的发育性神经毒性的有希望的治疗靶点。化学抑制剂渥曼青霉素对自噬的抑制和Atg7的siRNA敲低逆转了PBDE-47产生的有害结果。有趣的是,尽管与自噬抑制剂联合使用并不能进一步促进细胞存活,但是胱天蛋白酶3抑制剂Ac-DEVD-CHO对细胞凋亡的阻断改善了PBDE-47所致的自噬损伤和细胞死亡。结论:我们的数据表明自噬损伤促进细胞凋亡,继而破坏自噬,最终导致细胞死亡,并且自噬可能成为PBDE-47诱导的发育性神经毒性的有希望的治疗靶点。化学抑制剂渥曼青霉素对自噬的抑制和Atg7的siRNA敲低逆转了PBDE-47产生的有害结果。有趣的是,尽管与自噬抑制剂联合使用并不能进一步促进细胞存活,但是胱天蛋白酶3抑制剂Ac-DEVD-CHO对细胞凋亡的阻断改善了PBDE-47所致的自噬损伤和细胞死亡。结论:我们的数据表明自噬损伤促进细胞凋亡,继而破坏自噬,最终导致细胞死亡,并且自噬可能成为PBDE-47诱导的发育性神经毒性的有希望的治疗靶点。caspase-3抑制剂Ac-DEVD-CHO阻滞凋亡可改善PBDE-47引起的自噬损伤和细胞死亡,尽管与自噬抑制剂联合使用不会进一步促进细胞存活。结论:我们的数据表明自噬损伤促进细胞凋亡,继而破坏自噬,最终导致细胞死亡,并且自噬可能成为PBDE-47诱导的发育性神经毒性的有希望的治疗靶点。caspase-3抑制剂Ac-DEVD-CHO阻滞凋亡可改善PBDE-47引起的自噬损伤和细胞死亡,尽管与自噬抑制剂联合使用不会进一步促进细胞存活。结论:我们的数据表明自噬损伤促进细胞凋亡,继而破坏自噬,最终导致细胞死亡,并且自噬可能成为PBDE-47诱导的发育性神经毒性的有希望的治疗靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号