当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Towards measuring reactivity on micro-to-millisecond timescales with laser pump, NMR probe spectroscopy.
Faraday Discussions ( IF 3.4 ) Pub Date : 2019-12-02 , DOI: 10.1039/c9fd00039a Meghan E Halse 1 , Barbara Procacci , Robin N Perutz , Simon B Duckett
Faraday Discussions ( IF 3.4 ) Pub Date : 2019-12-02 , DOI: 10.1039/c9fd00039a Meghan E Halse 1 , Barbara Procacci , Robin N Perutz , Simon B Duckett
Affiliation
|
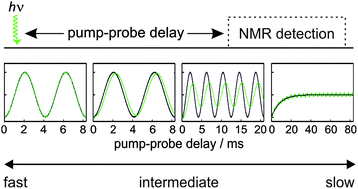
We present a quantitative analysis of the timescales of reactivity that are accessible to a laser pump, NMR probe spectroscopy method using para-hydrogen induced polarisation (PHIP) and identify three kinetic regimes: fast, intermediate and slow. These regimes are defined by the relative rate of reaction, k, compared to δω, the frequency of the NMR signal oscillations associated with the coherent evolution of the hyperpolarised 1H NMR signals created after para-hydrogen (p-H2) addition during the pump-probe delay. The kinetic regimes are quantitatively defined by a NMR dephasing parameter, ε = δω/k. For the fast regime, where k ≫ δω and ε tends to zero, the observed NMR signals are not affected by the chemical evolution of the system and so only an upper bound on k can be determined. In the slow regime, where k ≪ δω and ε tends to infinity, destructive interference leads to the complete dephasing of the coherent NMR signal intensity oscillations. As a result, the observed NMR signal evolution during the pump-probe delay reflects only the chemical change of the system and NMR relaxation. Finally, in the intermediate regime, where k ∼ δω, characteristic partial dephasing of the NMR signal oscillations is predicted. In the limit where the dephasing parameter is small but non-zero, chemical evolution manifests itself as a phase shift in the NMR signal oscillation that is equal to the dephasing parameter. As this phase shift is predicted to persist for pump-probe delays much longer than the timescale of the formation of the product molecules, it provides a route to measure reactivity on micro-to-millisecond timescales through NMR detection. We predict that the most significant fundamental limitations of the accessible reaction timescales are the duration of the NMR excitation pulse (∼1 μs) and the chemical shift difference (in Hz) between the p-H2-derived protons in the product molecule.
中文翻译:

为了通过激光泵,NMR探针光谱法在微秒至毫秒级上测量反应性。
我们对激光泵可访问的反应时间尺度进行定量分析,使用对氢诱导极化(PHIP)的NMR探针光谱法确定三种动力学机制:快速,中速和慢速。这些机制由相对反应速率k(与δω相比),与在泵浦过程中添加对氢(p-H2)后产生的超极化1H NMR信号的相干演化相关的NMR信号振荡频率定义。探测延迟。动力学方案由NMR移相参数ε=δω/ k定量定义。对于kδωω和ε趋于零的快速态,观察到的NMR信号不受系统化学演化的影响,因此只能确定k的上限。在慢速状态下,k≪δω和ε趋于无穷大,破坏性干扰导致相干NMR信号强度振荡的完全消相。结果,在泵浦探测延迟期间观察到的NMR信号演变仅反映了系统的化学变化和NMR弛豫。最后,在中间条件下,其中k〜δω,可以预测NMR信号振荡的特征性部分移相。在移相参数很小但不为零的极限中,化学演化本身表现为NMR信号振荡中的相移,该相移等于移相参数。由于预测该相移将持续比泵浦探针延迟持续更长的时间,而延迟时间远长于产物分子形成的时间尺度,因此它提供了一种通过NMR检测来测量微毫秒级反应性的途径。
更新日期:2019-12-04
中文翻译:

为了通过激光泵,NMR探针光谱法在微秒至毫秒级上测量反应性。
我们对激光泵可访问的反应时间尺度进行定量分析,使用对氢诱导极化(PHIP)的NMR探针光谱法确定三种动力学机制:快速,中速和慢速。这些机制由相对反应速率k(与δω相比),与在泵浦过程中添加对氢(p-H2)后产生的超极化1H NMR信号的相干演化相关的NMR信号振荡频率定义。探测延迟。动力学方案由NMR移相参数ε=δω/ k定量定义。对于kδωω和ε趋于零的快速态,观察到的NMR信号不受系统化学演化的影响,因此只能确定k的上限。在慢速状态下,k≪δω和ε趋于无穷大,破坏性干扰导致相干NMR信号强度振荡的完全消相。结果,在泵浦探测延迟期间观察到的NMR信号演变仅反映了系统的化学变化和NMR弛豫。最后,在中间条件下,其中k〜δω,可以预测NMR信号振荡的特征性部分移相。在移相参数很小但不为零的极限中,化学演化本身表现为NMR信号振荡中的相移,该相移等于移相参数。由于预测该相移将持续比泵浦探针延迟持续更长的时间,而延迟时间远长于产物分子形成的时间尺度,因此它提供了一种通过NMR检测来测量微毫秒级反应性的途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号