Cell Discovery ( IF 13.0 ) Pub Date : 2019-06-04 , DOI: 10.1038/s41421-019-0095-9 Yiqiang Zhang , Nuria Gago-Lopez , Ning Li , Zhenhe Zhang , Naima Alver , Yonggang Liu , Amy M. Martinson , Avin Mehri , William Robb MacLellan
|
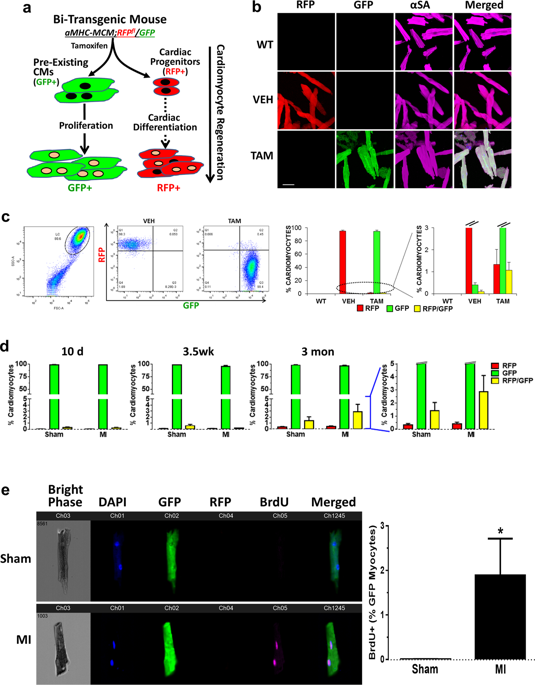
While it is recognized that there are low levels of new cardiomyocyte (CM) formation throughout life, the source of these new CM generates much debate. One hypothesis is that these new CMs arise from the proliferation of existing CMs potentially after dedifferentiation although direct evidence for this is lacking. Here we explore the mechanisms responsible for CM renewal in vivo using multi-reporter transgenic mouse models featuring efficient adult CM (ACM) genetic cell fate mapping and real-time cardiomyocyte lineage and dedifferentiation reporting. Our results demonstrate that non-myocytes (e.g., cardiac progenitor cells) contribute negligibly to new ACM formation at baseline or after cardiac injury. In contrast, we found a significant increase in dedifferentiated, cycling CMs in post-infarct hearts. ACM cell cycling was enhanced within the dedifferentiated CM population. Single-nucleus transcriptomic analysis demonstrated that CMs identified with dedifferentiation reporters had significant down-regulation in gene networks for cardiac hypertrophy, contractile, and electrical function, with shifts in metabolic pathways, but up-regulation in signaling pathways and gene sets for active cell cycle, proliferation, and cell survival. The results demonstrate that dedifferentiation may be an important prerequisite for CM proliferation and explain the limited but measurable cardiac myogenesis seen after myocardial infarction (MI).
中文翻译:

内源性心肌细胞去分化和循环的单细胞成像和转录组学分析
尽管人们认识到一生中新心肌细胞(CM)的形成水平很低,但这些新CM的来源引起了很多争论。一种假设是,尽管缺乏直接证据,这些新的CM可能来自去分化后现有CM的增殖。在这里,我们探讨了使用多报告人转基因小鼠模型负责体内CM更新的机制,这些模型具有有效的成年CM(ACM)遗传细胞命运定位以及实时心肌细胞谱系和去分化报告的功能。我们的结果表明,非心肌细胞(例如,心脏祖细胞)在基线或心脏损伤后对新的ACM形成的贡献可忽略不计。相反,我们发现梗死后心脏中未分化的循环CM显着增加。在去分化的CM群体中,ACM细胞循环增强。单核转录组学分析表明,用去分化报告基因鉴定的CM在心脏肥大,收缩和电功能基因网络中显着下调,代谢途径发生改变,但在信号途径和活跃细胞周期的基因组中上调,增殖和细胞存活率。结果表明,去分化可能是CM增殖的重要先决条件,并解释了心肌梗死(MI)后所见的有限但可测量的心肌发生。和电子功能,但代谢途径有所改变,但信号传导途径和基因集的上调却活跃了细胞的周期,增殖和细胞存活。结果表明,去分化可能是CM增殖的重要先决条件,并解释了心肌梗死(MI)后所见的有限但可测量的心肌发生。和电子功能,但代谢途径有所改变,但信号传导途径和基因集的上调却活跃了细胞的周期,增殖和细胞存活。结果表明,去分化可能是CM增殖的重要先决条件,并解释了心肌梗死(MI)后所见的有限但可测量的心肌发生。










































 京公网安备 11010802027423号
京公网安备 11010802027423号