当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
mGlu5 Positive Allosteric Modulators Facilitate Long-Term Potentiation via Disinhibition Mediated by mGlu5-Endocannabinoid Signaling.
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-05-15 , DOI: 10.1021/acsptsci.9b00017 Zixiu Xiang 1 , Xiaohui Lv 1 , James Maksymetz 1 , Branden J Stansley 1 , Ayan Ghoshal 1 , Rocco G Gogliotti 1 , Colleen M Niswender 1 , Craig W Lindsley 1 , P Jeffrey Conn 1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-05-15 , DOI: 10.1021/acsptsci.9b00017 Zixiu Xiang 1 , Xiaohui Lv 1 , James Maksymetz 1 , Branden J Stansley 1 , Ayan Ghoshal 1 , Rocco G Gogliotti 1 , Colleen M Niswender 1 , Craig W Lindsley 1 , P Jeffrey Conn 1
Affiliation
|
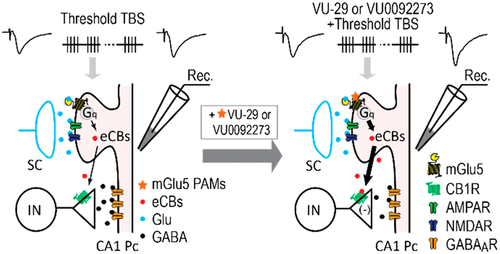
Metabotropic glutamate (mGlu) receptor type 5 (mGlu5) positive allosteric modulators (PAMs) enhance hippocampal long-term potentiation (LTP) and have cognition-enhancing effects in animal models. These effects were initially thought to be mediated by potentiation of mGlu5 modulation of N-methyl-d-aspartate receptor (NMDAR) currents. However, a biased mGlu5 PAM that potentiates Gαq-dependent mGlu5 signaling, but not mGlu5 modulation of NMDAR currents, retains cognition-enhancing effects in animal models, suggesting that potentiation of NMDAR currents is not required for these in vivo effects of mGlu5 PAMs. However, it is not clear whether the potentiation of NMDAR currents is critical for the ability of mGlu5 PAMs to enhance hippocampal LTP. We now report the characterization of effects of two structurally distinct mGlu5 PAMs, VU-29 and VU0092273, on NMDAR currents and hippocampal LTP. As with other mGlu5 PAMs that do not display observable bias for potentiation of NMDAR currents, VU0092273 enhanced both mGlu5 modulation of NMDAR currents and induction of LTP at the hippocampal Schaffer collateral (SC)-CA1 synapse. In contrast, VU-29 did not potentiate mGlu5 modulation of NMDAR currents but induced robust potentiation of hippocampal LTP. Interestingly, both VU-29 and VU0092273 suppressed evoked inhibitory postsynaptic currents (eIPSCs) in CA1 pyramidal cells, and this effect was blocked by the cannabinoid receptor type 1 (CB1) antagonist AM251. Furthermore, AM251 blocked the ability of both mGlu5 PAMs to enhance LTP. Finally, both PAMs failed to enhance LTP in mice with the restricted genetic deletion of mGlu5 in CA1 pyramidal cells. Taken together with previous findings, these results suggest that enhancement of LTP by mGlu5 PAMs does not depend on mGlu5 modulation of NMDAR currents but is mediated by a previously established mechanism in which mGlu5 in CA1 pyramidal cells induces endocannabinoid release and CB1-dependent disinhibition.
中文翻译:

mGlu5阳性变构调节剂通过mGlu5-内源性大麻素信号传导介导的抑制作用促进长期增强。
代谢型谷氨酸(mGlu)受体5型(mGlu5)阳性变构调节剂(PAM)增强海马长期增强(LTP)并在动物模型中具有认知增强作用。最初认为这些作用是由增强N-甲基-d-天冬氨酸受体(NMDAR)电流的mGlu5调节介导的。但是,有偏见的mGlu5 PAM可以增强Gαq依赖性mGlu5信号传导,但不能增强NMDAR电流的mGlu5调节,在动物模型中保留了认知增强作用,这表明mGlu5 PAM的这些体内效应不需要NMDAR电流的增强。但是,尚不清楚NMDAR电流的增强对于mGlu5 PAM增强海马LTP的能力是否至关重要。现在,我们报告两种结构上不同的mGlu5 PAM,VU-29和VU0092273的作用表征,NMDAR电流和海马LTP的影响。与其他对NMDAR电流没有明显影响的mGlu5 PAM一样,VU0092273增强了海马Schaffer侧支(SC)-CA1突触对NMDAR电流的mGlu5调制和LTP诱导。相反,VU-29不能增强NMDAR电流的mGlu5调节,但可以诱导海马LTP的强大增强作用。有趣的是,VU-29和VU0092273均抑制了CA1锥体细胞中诱发的抑制性突触后电流(eIPSC),并且这种作用被1型大麻素受体(CB1)拮抗剂AM251阻断。此外,AM251阻止了两个mGlu5 PAM增强LTP的能力。最后,由于PA1锥体细胞中mGlu5基因的限制性遗传缺失,两个PAM均未能增强小鼠的LTP。结合以前的发现,
更新日期:2019-06-03
中文翻译:

mGlu5阳性变构调节剂通过mGlu5-内源性大麻素信号传导介导的抑制作用促进长期增强。
代谢型谷氨酸(mGlu)受体5型(mGlu5)阳性变构调节剂(PAM)增强海马长期增强(LTP)并在动物模型中具有认知增强作用。最初认为这些作用是由增强N-甲基-d-天冬氨酸受体(NMDAR)电流的mGlu5调节介导的。但是,有偏见的mGlu5 PAM可以增强Gαq依赖性mGlu5信号传导,但不能增强NMDAR电流的mGlu5调节,在动物模型中保留了认知增强作用,这表明mGlu5 PAM的这些体内效应不需要NMDAR电流的增强。但是,尚不清楚NMDAR电流的增强对于mGlu5 PAM增强海马LTP的能力是否至关重要。现在,我们报告两种结构上不同的mGlu5 PAM,VU-29和VU0092273的作用表征,NMDAR电流和海马LTP的影响。与其他对NMDAR电流没有明显影响的mGlu5 PAM一样,VU0092273增强了海马Schaffer侧支(SC)-CA1突触对NMDAR电流的mGlu5调制和LTP诱导。相反,VU-29不能增强NMDAR电流的mGlu5调节,但可以诱导海马LTP的强大增强作用。有趣的是,VU-29和VU0092273均抑制了CA1锥体细胞中诱发的抑制性突触后电流(eIPSC),并且这种作用被1型大麻素受体(CB1)拮抗剂AM251阻断。此外,AM251阻止了两个mGlu5 PAM增强LTP的能力。最后,由于PA1锥体细胞中mGlu5基因的限制性遗传缺失,两个PAM均未能增强小鼠的LTP。结合以前的发现,











































 京公网安备 11010802027423号
京公网安备 11010802027423号