当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gadolinium-Chelated Conjugated Polymer-Based Nanotheranostics for Photoacoustic/Magnetic Resonance/NIR-II Fluorescence Imaging-Guided Cancer Photothermal Therapy.
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.34390 Xiaoming Hu 1 , Yufu Tang 1 , Yuxuan Hu 2 , Feng Lu 1 , Xiaomei Lu 3 , Yuqi Wang 2 , Jie Li 1 , Yuanyuan Li 1 , Yu Ji 1 , Wenjun Wang 4 , Deju Ye 2 , Quli Fan 1 , Wei Huang 1, 3, 5
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.34390 Xiaoming Hu 1 , Yufu Tang 1 , Yuxuan Hu 2 , Feng Lu 1 , Xiaomei Lu 3 , Yuqi Wang 2 , Jie Li 1 , Yuanyuan Li 1 , Yu Ji 1 , Wenjun Wang 4 , Deju Ye 2 , Quli Fan 1 , Wei Huang 1, 3, 5
Affiliation
|
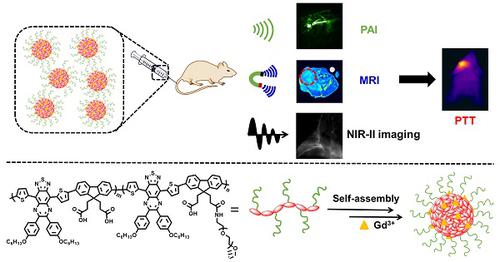
Our exploiting versatile multimodal theranostic agent aims to integrate the complementary superiorities of photoacoustic imaging (PAI), second near-infrared (NIR-II, 1000-1700) fluorescence and T1-weighted magnetic resonance imaging (MRI) with an ultimate objective of perfecting cancer diagnosis, thus improving cancer therapy efficacy. Herein, we engineered and prepared a water-soluble gadolinium-chelated conjugated polymer-based theranostic nanomedicine (PFTQ-PEG-Gd NPs) for in vivo tri-mode PA/MR/NIR-II imaging-guided tumor photothermal therapy (PTT). Methods: We firstly constructed a semiconducting polymer composed of low-bandgap donor-acceptor (D-A) which afforded the strong NIR absorption for PAI/PTT and long fluorescence emission to NIR-II region for in vivo imaging. Then, the remaining carboxyl groups of the polymeric NPs could effectively chelate with Gd3+ ions for MRI. The in vitro characteristics of the PFTQ-PEG-Gd NPs were studied and the in vivo multimode imaging as well as anti-tumor efficacy of the NPs was evaluated using 4T1 tumor-bearing mice. Results: The obtained theranostic agent showed excellent chemical and optical stability as well as low biotoxicity. After 24 h of systemic administration using PQTF-PEG-Gd NPs, the tumor sites of living mice exhibited obvious enhancement in PA, NIR-II fluorescence and positive MR signal intensities. Better still, a conspicuous tumor growth restraint was detected under NIR light irradiation after administration of PQTF-PEG-Gd NPs, indicating the efficient photothermal potency of the nano-agent. Conclusion: we triumphantly designed and synthesized a novel and omnipotent semiconducting polymer nanoparticles-based theranostic platform for PAI, NIR-II fluorescence imaging as well as positive MRI-guided tumor PTT in living mice. We expect that such a novel organic nano-platform manifests a great promise for high spatial resolution and deep penetration cancer theranostics.
中文翻译:

d螯合共轭聚合物基纳米热敏材料,用于光声/磁共振/ NIR-II荧光成像引导的癌症光热疗法。
我们开发的多功能多峰治疗药物旨在将光声成像(PAI),第二次近红外(NIR-II,1000-1700)荧光和T1加权磁共振成像(MRI)的互补优势与完善癌症的最终目标相结合诊断,从而提高癌症治疗的疗效。在这里,我们设计和制备了一种水溶性g螯合的共轭聚合物基治疗药物纳米药物(PFTQ-PEG-Gd NPs),用于体内三模PA / MR / NIR-II成像引导的肿瘤光热疗法(PTT)。方法:我们首先构建了一种由低带隙供体-受体(DA)组成的半导体聚合物,该聚合物对PAI / PTT具有很强的NIR吸收能力,并且可以在体内成像时向NIR-II区提供长时间的荧光发射。然后,聚合物NP的其余羧基可以与Gd3 +离子有效螯合进行MRI检查。研究了PFTQ-PEG-Gd NP的体外特性,并使用4T1荷瘤小鼠评估了NPs的体内多模成像以及抗肿瘤功效。结果:所获得的治疗药物具有优异的化学和光学稳定性以及低的生物毒性。使用PQTF-PEG-Gd NP全身性给药24小时后,活体小鼠的肿瘤部位显示出PA,NIR-II荧光增强和MR信号强度阳性的明显增强。更好的是,在施用PQTF-PEG-Gd NP后,在NIR光照射下检测到明显的肿瘤生长抑制作用,表明了纳米剂的有效光热效能。结论:我们成功地设计和合成了一种新型的,全能的基于聚合物纳米粒子的半导体治疗学平台,用于活体小鼠的PAI,NIR-II荧光成像以及MRI指导的阳性肿瘤PTT。我们期望这种新颖的有机纳米平台对高空间分辨率和深度渗透癌症治疗学表现出巨大的希望。
更新日期:2019-01-01
中文翻译:

d螯合共轭聚合物基纳米热敏材料,用于光声/磁共振/ NIR-II荧光成像引导的癌症光热疗法。
我们开发的多功能多峰治疗药物旨在将光声成像(PAI),第二次近红外(NIR-II,1000-1700)荧光和T1加权磁共振成像(MRI)的互补优势与完善癌症的最终目标相结合诊断,从而提高癌症治疗的疗效。在这里,我们设计和制备了一种水溶性g螯合的共轭聚合物基治疗药物纳米药物(PFTQ-PEG-Gd NPs),用于体内三模PA / MR / NIR-II成像引导的肿瘤光热疗法(PTT)。方法:我们首先构建了一种由低带隙供体-受体(DA)组成的半导体聚合物,该聚合物对PAI / PTT具有很强的NIR吸收能力,并且可以在体内成像时向NIR-II区提供长时间的荧光发射。然后,聚合物NP的其余羧基可以与Gd3 +离子有效螯合进行MRI检查。研究了PFTQ-PEG-Gd NP的体外特性,并使用4T1荷瘤小鼠评估了NPs的体内多模成像以及抗肿瘤功效。结果:所获得的治疗药物具有优异的化学和光学稳定性以及低的生物毒性。使用PQTF-PEG-Gd NP全身性给药24小时后,活体小鼠的肿瘤部位显示出PA,NIR-II荧光增强和MR信号强度阳性的明显增强。更好的是,在施用PQTF-PEG-Gd NP后,在NIR光照射下检测到明显的肿瘤生长抑制作用,表明了纳米剂的有效光热效能。结论:我们成功地设计和合成了一种新型的,全能的基于聚合物纳米粒子的半导体治疗学平台,用于活体小鼠的PAI,NIR-II荧光成像以及MRI指导的阳性肿瘤PTT。我们期望这种新颖的有机纳米平台对高空间分辨率和深度渗透癌症治疗学表现出巨大的希望。











































 京公网安备 11010802027423号
京公网安备 11010802027423号