当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toward Defining the Pharmacophore for Positive Allosteric Modulation of PTH1 Receptor Signaling by Extracellular Nucleotides.
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-05-29 , DOI: 10.1021/acsptsci.8b00053 Brandon H Kim 1 , Fang I Wang 1 , Alexey Pereverzev 1 , Peter Chidiac 1 , S Jeffrey Dixon 1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-05-29 , DOI: 10.1021/acsptsci.8b00053 Brandon H Kim 1 , Fang I Wang 1 , Alexey Pereverzev 1 , Peter Chidiac 1 , S Jeffrey Dixon 1
Affiliation
|
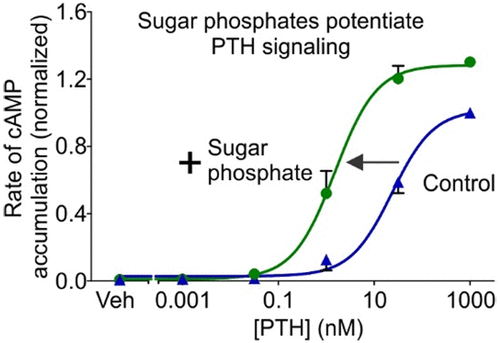
The parathyroid hormone 1 receptor (PTH1R) is a Class B G-protein-coupled receptor that is a target for osteoporosis therapeutics. Activated PTH1R couples through Gs to the stimulation of adenylyl cyclase. As well, β-arrestin is recruited to PTH1R leading to receptor internalization and MAPK/ERK signaling. Previously, we reported that the agonist potency of PTH1R is increased in the presence of extracellular ATP, which acts as a positive allosteric modulator of PTH signaling. Another nucleotide, cytidine 5'-monophosphate (CMP), also enhances PTH1R signaling, suggesting that ATP and CMP share a moiety responsible for positive allostery, possibly ribose-5-phosphate. Therefore, we examined the effect of extracellular sugar phosphates on PTH1R signaling. cAMP levels and β-arrestin recruitment were monitored using luminescence-based assays. Alone, ribose-5-phosphate had no detectable effect on adenylyl cyclase activity in UMR-106 rat osteoblastic cells, which endogenously express PTH1R. However, ribose-5-phosphate markedly enhanced the activation of adenylyl cyclase induced by PTH. Other sugar phosphates, including glucose-1-phosphate, glucose-6-phosphate, fructose-6-phosphate, and fructose-1,6-bisphosphate, also potentiated PTH-induced adenylyl cyclase activation. As well, some sugar phosphates enhanced PTH-induced β-arrestin recruitment to human PTH1R heterologously expressed in HEK293H cells. Interestingly, the effects of glucose-1-phosphate were greater than those of its isomer glucose-6-phosphate. Our results suggest that phosphorylated monosaccharides such as ribose-5-phosphate contain the pharmacophore for positive allosteric modulation of PTH1R. At least in some cases, the extent of modulation depends on the position of the phosphate group. Knowledge of the pharmacophore may permit future development of positive allosteric modulators to increase the therapeutic efficacy of PTH1R agonists.
中文翻译:

旨在定义药理作用的细胞外核苷酸对PTH1受体信号的正向变构调节。
甲状旁腺激素1受体(PTH1R)是B类G蛋白偶联受体,是骨质疏松症治疗的靶标。激活的PTH1R通过Gs偶联至腺苷酸环化酶的刺激。同样,β-arrestin被募集到PTH1R,从而导致受体内在化和MAPK / ERK信号传导。以前,我们报道了PTH1R的激动剂效力在细胞外ATP的存在下增加,而细胞外ATP则是PTH信号的正变构调节剂。另一个核苷酸胞苷5'-单磷酸酯(CMP)也增强了PTH1R信号传导,表明ATP和CMP共有负责阳性变构的部分,可能是5磷酸核糖。因此,我们检查了细胞外糖磷酸酯对PTH1R信号传导的影响。使用基于发光的测定法监测cAMP水平和β-arrestin募集。独自的,5核糖对内源性表达PTH1R的UMR-106大鼠成骨细胞中的腺苷酸环化酶活性没有可检测的影响。然而,核糖-5-磷酸显着增强了由PTH诱导的腺苷酸环化酶的活化。其他糖磷酸酯,包括-1-磷酸葡萄糖,6-磷酸葡萄糖,6-磷酸果糖和-1,6-二磷酸果糖,也可以增强PTH诱导的腺苷酸环化酶激活。同样,一些糖磷酸酯增强了PTH诱导的β-arrestin募集至在HEK293H细胞中异源表达的人PTH1R。有趣的是,葡萄糖-1-磷酸的作用大于异构体葡萄糖6-磷酸的作用。我们的结果表明,磷酸化的单糖(如核糖5-磷酸)含有药效基团,可对PTH1R进行正构构调节。至少在某些情况下,调节程度取决于磷酸基团的位置。药效基团的知识可能允许将来发展正构构调节剂,以增加PTH1R激动剂的治疗功效。
更新日期:2019-05-31
中文翻译:

旨在定义药理作用的细胞外核苷酸对PTH1受体信号的正向变构调节。
甲状旁腺激素1受体(PTH1R)是B类G蛋白偶联受体,是骨质疏松症治疗的靶标。激活的PTH1R通过Gs偶联至腺苷酸环化酶的刺激。同样,β-arrestin被募集到PTH1R,从而导致受体内在化和MAPK / ERK信号传导。以前,我们报道了PTH1R的激动剂效力在细胞外ATP的存在下增加,而细胞外ATP则是PTH信号的正变构调节剂。另一个核苷酸胞苷5'-单磷酸酯(CMP)也增强了PTH1R信号传导,表明ATP和CMP共有负责阳性变构的部分,可能是5磷酸核糖。因此,我们检查了细胞外糖磷酸酯对PTH1R信号传导的影响。使用基于发光的测定法监测cAMP水平和β-arrestin募集。独自的,5核糖对内源性表达PTH1R的UMR-106大鼠成骨细胞中的腺苷酸环化酶活性没有可检测的影响。然而,核糖-5-磷酸显着增强了由PTH诱导的腺苷酸环化酶的活化。其他糖磷酸酯,包括-1-磷酸葡萄糖,6-磷酸葡萄糖,6-磷酸果糖和-1,6-二磷酸果糖,也可以增强PTH诱导的腺苷酸环化酶激活。同样,一些糖磷酸酯增强了PTH诱导的β-arrestin募集至在HEK293H细胞中异源表达的人PTH1R。有趣的是,葡萄糖-1-磷酸的作用大于异构体葡萄糖6-磷酸的作用。我们的结果表明,磷酸化的单糖(如核糖5-磷酸)含有药效基团,可对PTH1R进行正构构调节。至少在某些情况下,调节程度取决于磷酸基团的位置。药效基团的知识可能允许将来发展正构构调节剂,以增加PTH1R激动剂的治疗功效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号