当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering temperature-sensitive plateletsomes as a tailored chemotherapy platform in combination with HIFU ablation for cancer treatment.
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.32172 Dongqi Wu 1 , Xing Jin 1 , Xiaobing Wang 1 , Boyu Ma 1 , Chenmei Lou 1 , Haijing Qu 1 , Jian Zheng 1 , Binxuan Zhang 1 , Xiufeng Yan 1 , Yang Wang 1 , Lijia Jing 1
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.32172 Dongqi Wu 1 , Xing Jin 1 , Xiaobing Wang 1 , Boyu Ma 1 , Chenmei Lou 1 , Haijing Qu 1 , Jian Zheng 1 , Binxuan Zhang 1 , Xiufeng Yan 1 , Yang Wang 1 , Lijia Jing 1
Affiliation
|
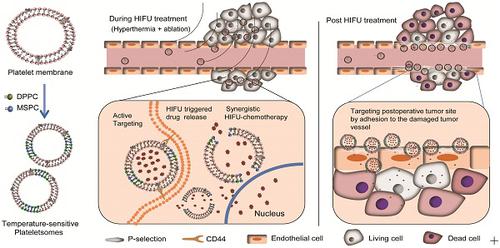
Chemotherapy is widely used in combination with high-intensity focused ultrasound (HIFU) ablation for cancer therapy; however, the spatial and temporal integration of chemotherapy and HIFU ablation remains a challenge. Here, temperature-sensitive plateletsomes (TSPs) composed of platelet (PLT) membrane, 1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine were developed to adequately integrate chemotherapy with HIFU tumor ablation in vivo. Methods: The thermosensitive permeability of TSPs was evaluated under both water bath heating and HIFU hyperthermia. The targeting performance, pharmacokinetic behavior and therapeutic potential of TSPs in combination with HIFU ablation were evaluated using HeLa cells and a HeLa cell tumor-bearing nude mouse model in comparison with temperature-sensitive liposomes (TSLs). Results: TSPs showed high drug loading efficiency and temperature-sensitive permeability. When applied in vivo, TSPs showed a circulation lifetime comparable to that of TSLs and exhibited PLT-specific cancer cell affinity and a vascular damage response. Upon HIFU hyperthermia, TSPs displayed ultrafast drug release and enhanced tumor uptake, providing high drug availability in the tumor site to cooperate with HIFU ablation. After HIFU ablation, TSPs rapidly targeted the postoperative tumor site by adhesion to the damaged tumor vasculature, leading to targeted and localized postoperative chemotherapy. Conclusion: Due to effective integration at both intraoperative and postoperative stages, TSPs could be a promising chemotherapy nanoplatform in combination with HIFU ablation for cancer therapy.
中文翻译:

将温度敏感的血小板工程化为量身定制的化疗平台,结合HIFU消融治疗癌症。
化学疗法与高强度聚焦超声(HIFU)消融相结合广泛用于癌症治疗。然而,化学疗法和HIFU消融的时空整合仍然是一个挑战。在这里,由血小板(PLT)膜,1-硬脂酰基-2-羟基-sn-甘油-3-磷酸胆碱和1,2-二棕榈酰基-sn-甘油-3-磷酸胆碱组成的温度敏感血小板体(TSPs)已被充分开发。在体内将化疗与HIFU肿瘤消融相结合。方法:在水浴加热和HIFU高温下评估TSPs的热敏渗透性。定位效果 与温度敏感脂质体(TSLs)相比,使用HeLa细胞和带有HeLa细胞瘤的裸鼠模型评估了TSPs结合HIFU消融的药代动力学行为和治疗潜力。结果:TSPs具有较高的载药效率和对温度敏感的渗透性。当在体内应用时,TSPs的循环寿命可与TSL相当,并且具有PLT特异性癌细胞亲和力和血管损伤反应。HIFU热疗后,TSP显示出超快的药物释放和增强的肿瘤吸收能力,从而在肿瘤部位提供了较高的药物利用率,可与HIFU消融合作。HIFU消融后,TSP通过粘附到受损的肿瘤血管上而迅速靶向术后肿瘤部位,从而导致靶向和局部术后化疗。结论:
更新日期:2019-01-01
中文翻译:

将温度敏感的血小板工程化为量身定制的化疗平台,结合HIFU消融治疗癌症。
化学疗法与高强度聚焦超声(HIFU)消融相结合广泛用于癌症治疗。然而,化学疗法和HIFU消融的时空整合仍然是一个挑战。在这里,由血小板(PLT)膜,1-硬脂酰基-2-羟基-sn-甘油-3-磷酸胆碱和1,2-二棕榈酰基-sn-甘油-3-磷酸胆碱组成的温度敏感血小板体(TSPs)已被充分开发。在体内将化疗与HIFU肿瘤消融相结合。方法:在水浴加热和HIFU高温下评估TSPs的热敏渗透性。定位效果 与温度敏感脂质体(TSLs)相比,使用HeLa细胞和带有HeLa细胞瘤的裸鼠模型评估了TSPs结合HIFU消融的药代动力学行为和治疗潜力。结果:TSPs具有较高的载药效率和对温度敏感的渗透性。当在体内应用时,TSPs的循环寿命可与TSL相当,并且具有PLT特异性癌细胞亲和力和血管损伤反应。HIFU热疗后,TSP显示出超快的药物释放和增强的肿瘤吸收能力,从而在肿瘤部位提供了较高的药物利用率,可与HIFU消融合作。HIFU消融后,TSP通过粘附到受损的肿瘤血管上而迅速靶向术后肿瘤部位,从而导致靶向和局部术后化疗。结论:











































 京公网安备 11010802027423号
京公网安备 11010802027423号