当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Clathrin-mediated Endocytosis of Alpha-1 Antitrypsin is Essential for its Protective Function in Islet Cell Survival.
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.31647 Jingjing Wang 1 , Wenyu Gou 1 , Do-Sung Kim 1 , Charlie Strange 2 , Hongjun Wang 1, 3
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.31647 Jingjing Wang 1 , Wenyu Gou 1 , Do-Sung Kim 1 , Charlie Strange 2 , Hongjun Wang 1, 3
Affiliation
|
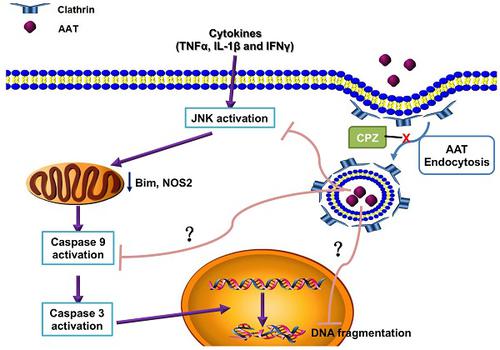
Cytokine-induced pancreatic β cell death plays a pivotal role in both type 1 and type 2 diabetes. Our previous study showed that alpha-1 antitrypsin (AAT) inhibits β cell death through the suppression of cytokine-induced c-Jun N-terminal kinase (JNK) activation in an islet transplantation model. The aim of this study was to further understand how AAT impacts β cells by studying AAT endocytosis in human islets and a βTC3 murine insulinoma cell line. Methods: In vitro, human islets and βTC3 cells were stimulated with cytokines in the presence or absence of chlorpromazine (CPZ), a drug that disrupts clathrin-mediated endocytosis. Western blot, real-time PCR and cell death ELISA were performed to investigate β cell death. The oxygen consumption rate (OCR) was measured on human islets. In vivo, islets were harvested from C57BL/6 donor mice treated with saline or human AAT and transplanted into the livers of syngeneic mice that had been rendered diabetic by streptozotocin (STZ). Islet graft survival and function were analyzed. Results: AAT was internalized by β cells in a time- and dose-dependent manner. AAT internalization was mediated by clathrin as treatment with CPZ, profoundly decreased AAT internalization, cytokine-induced JNK activation and the downstream upregulation of c-Jun mRNA expression. Similarly, addition of CPZ attenuated cytokine-induced caspase 9 cleavage (c-casp 9) and DNA fragmentation, which was suppressed by AAT. Treatment of donor mice with AAT produced AAT internalization in islets, and resulted in a higher percentage of recipients reaching normoglycemia after syngeneic intraportal islet transplantation. Conclusion: Our results suggest that AAT is internalized by β cells through clathrin-mediated endocytosis that leads to the suppression of caspase 9 activation. This process is required for the protective function of AAT in islets when challenged with proinflammatory cytokines or after islet transplantation.
中文翻译:

网格蛋白介导的Alpha-1抗胰蛋白酶的内吞作用对其在胰岛细胞存活中的保护功能至关重要。
细胞因子诱导的胰腺β细胞死亡在1型和2型糖尿病中均起着关键作用。我们先前的研究表明,在胰岛移植模型中,α-1抗胰蛋白酶(AAT)通过抑制细胞因子诱导的c-Jun N-末端激酶(JNK)激活来抑制β细胞死亡。这项研究的目的是通过研究人胰岛和βTC3鼠胰岛素瘤细胞系中的AAT内吞作用,进一步了解AAT如何影响β细胞。方法:在体外,在存在或不存在氯丙嗪(CPZ)的情况下,用细胞因子刺激人胰岛和βTC3细胞,该药物可破坏网格蛋白介导的内吞作用。进行了蛋白质印迹,实时PCR和细胞死亡ELISA来研究β细胞死亡。在人类胰岛上测量了耗氧率(OCR)。体内,从用盐水或人AAT处理过的C57BL / 6供体小鼠中收获胰岛,并将其移植到已被链脲佐菌素(STZ)赋予糖尿病的同系小鼠的肝脏中。分析胰岛移植物的存活和功能。结果:AAT被β细胞以时间和剂量依赖的方式内在化。网格蛋白作为CPZ处理可介导AAT内在化,从而大大降低AAT内在化,细胞因子诱导的JNK活化以及c-Jun mRNA表达的下游上调。同样,CPZ的添加减弱了细胞因子诱导的caspase 9裂解(c-casp 9)和DNA片段化,这被AAT抑制。用AAT治疗供体小鼠会在胰岛中产生AAT内在化,并导致同基因门静脉内胰岛移植后达到正常血糖的接受者比例更高。结论:我们的结果表明AAT被网格蛋白介导的内吞作用的β细胞内在化,从而导致caspase 9激活的抑制。当用促炎细胞因子攻击或胰岛移植后,此过程对于AAT在胰岛中的保护功能是必需的。
更新日期:2019-01-01
中文翻译:

网格蛋白介导的Alpha-1抗胰蛋白酶的内吞作用对其在胰岛细胞存活中的保护功能至关重要。
细胞因子诱导的胰腺β细胞死亡在1型和2型糖尿病中均起着关键作用。我们先前的研究表明,在胰岛移植模型中,α-1抗胰蛋白酶(AAT)通过抑制细胞因子诱导的c-Jun N-末端激酶(JNK)激活来抑制β细胞死亡。这项研究的目的是通过研究人胰岛和βTC3鼠胰岛素瘤细胞系中的AAT内吞作用,进一步了解AAT如何影响β细胞。方法:在体外,在存在或不存在氯丙嗪(CPZ)的情况下,用细胞因子刺激人胰岛和βTC3细胞,该药物可破坏网格蛋白介导的内吞作用。进行了蛋白质印迹,实时PCR和细胞死亡ELISA来研究β细胞死亡。在人类胰岛上测量了耗氧率(OCR)。体内,从用盐水或人AAT处理过的C57BL / 6供体小鼠中收获胰岛,并将其移植到已被链脲佐菌素(STZ)赋予糖尿病的同系小鼠的肝脏中。分析胰岛移植物的存活和功能。结果:AAT被β细胞以时间和剂量依赖的方式内在化。网格蛋白作为CPZ处理可介导AAT内在化,从而大大降低AAT内在化,细胞因子诱导的JNK活化以及c-Jun mRNA表达的下游上调。同样,CPZ的添加减弱了细胞因子诱导的caspase 9裂解(c-casp 9)和DNA片段化,这被AAT抑制。用AAT治疗供体小鼠会在胰岛中产生AAT内在化,并导致同基因门静脉内胰岛移植后达到正常血糖的接受者比例更高。结论:我们的结果表明AAT被网格蛋白介导的内吞作用的β细胞内在化,从而导致caspase 9激活的抑制。当用促炎细胞因子攻击或胰岛移植后,此过程对于AAT在胰岛中的保护功能是必需的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号