当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Forkhead box K2 promotes human colorectal cancer metastasis by upregulating ZEB1 and EGFR.
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.31716 Feng Du 1 , Chenyang Qiao 1 , Xiaowei Li 1, 2 , Zhangqian Chen 1, 3 , Hao Liu 1 , Shengda Wu 4 , Sijun Hu 1 , Zhaoyan Qiu 5 , Meirui Qian 1 , Dean Tian 6 , Kaichun Wu 1 , Daiming Fan 1 , Yongzhan Nie 1 , Limin Xia 1, 6
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.31716 Feng Du 1 , Chenyang Qiao 1 , Xiaowei Li 1, 2 , Zhangqian Chen 1, 3 , Hao Liu 1 , Shengda Wu 4 , Sijun Hu 1 , Zhaoyan Qiu 5 , Meirui Qian 1 , Dean Tian 6 , Kaichun Wu 1 , Daiming Fan 1 , Yongzhan Nie 1 , Limin Xia 1, 6
Affiliation
|
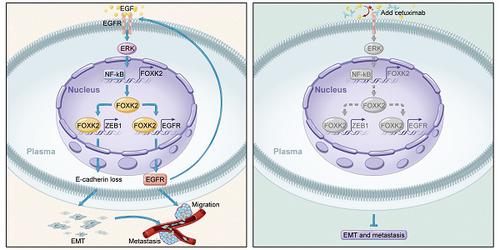
Background: Metastasis is the major reason for high recurrence rates and poor survival among patients with colorectal cancer (CRC). However, the underlying molecular mechanism of CRC metastasis is unclear. This study aimed to investigate the role of forkhead box K2 (FOXK2), one of the most markedly increased FOX genes in CRC, and the mechanism by which it is deregulated in CRC metastasis. Methods: FOXK2 levels were analyzed in two independent human CRC cohorts (cohort I, n = 363; cohort II, n = 390). In vitro Transwell assays and in vivo lung and liver metastasis models were used to examine CRC cell migration, invasion and metastasis. Chromatin immunoprecipitation and luciferase reporter assays were used to measure the binding of transcription factors to the promoters of FOXK2, zinc finger E-box binding homeobox 1 (ZEB1) and epidermal growth factor receptor (EGFR). Cetuximab was utilized to treat FOXK2-mediated metastatic CRC. Results: FOXK2 was significantly upregulated in human CRC tissues, was correlated with more aggressive features and indicated a poor prognosis. FOXK2 overexpression promoted CRC migration, invasion and metastasis, while FOXK2 downregulation had the opposite effects. ZEB1 and EGFR were determined to be direct transcriptional targets of FOXK2 and were essential for FOXK2-mediated CRC metastasis. Moreover, activation of EGFR signaling by EGF enhanced FOXK2 expression via the extracellular regulated protein kinase (ERK) and nuclear factor (NF)-κB pathways. The EGFR monoclonal antibody cetuximab significantly inhibited FOXK2-promoted CRC metastasis. In clinical CRC tissues, FOXK2 expression was positively correlated with the expression of p65, ZEB1 and EGFR. CRC patients who coexpressed p65/FOXK2, FOXK2/ZEB1 and FOXK2/EGFR had poorer prognosis. Conclusions: FOXK2 serves as a prognostic biomarker in CRC. Cetuximab can block the EGF-NF-κB-FOXK2-EGFR feedback loop and suppress CRC metastasis.
中文翻译:

前叉箱K2通过上调ZEB1和EGFR促进人类大肠癌转移。
背景:转移是结直肠癌(CRC)患者高复发率和低生存率的主要原因。但是,CRC转移的潜在分子机制尚不清楚。这项研究旨在研究叉头盒K2(FOXK2)(在CRC中最显着增加的FOX基因之一)的作用,以及它在CRC转移中被解除调节的机制。方法:在两个独立的人类CRC队列中分析了FOXK2水平(队列I,n = 363;队列II,n = 390)。体外Transwell测定法和体内肺和肝转移模型用于检查CRC细胞的迁移,侵袭和转移。染色质免疫沉淀和荧光素酶报告基因分析用于测量转录因子与FOXK2启动子的结合,锌指E-box结合同源异型盒1(ZEB1)和表皮生长因子受体(EGFR)。西妥昔单抗用于治疗FOXK2介导的转移性CRC。结果:FOXK2在人CRC组织中显着上调,与更具侵略性的特征相关,预后不良。FOXK2的过表达促进了CRC的迁移,侵袭和转移,而FOXK2的下调则具有相反的作用。ZEB1和EGFR被确定为FOXK2的直接转录目标,并且是FOXK2介导的CRC转移所必需的。此外,EGF对EGFR信号的激活通过细胞外调节蛋白激酶(ERK)和核因子(NF)-κB途径增强了FOXK2的表达。EGFR单克隆抗体西妥昔单抗显着抑制FOXK2促进的CRC转移。在临床CRC组织中,FOXK2表达与p65,ZEB1和EGFR的表达呈正相关。共表达p65 / FOXK2,FOXK2 / ZEB1和FOXK2 / EGFR的CRC患者预后较差。结论:FOXK2可作为CRC的预后生物标志物。西妥昔单抗可阻断EGF-NF-κB-FOXK2-EGFR反馈回路并抑制CRC转移。
更新日期:2019-01-01
中文翻译:

前叉箱K2通过上调ZEB1和EGFR促进人类大肠癌转移。
背景:转移是结直肠癌(CRC)患者高复发率和低生存率的主要原因。但是,CRC转移的潜在分子机制尚不清楚。这项研究旨在研究叉头盒K2(FOXK2)(在CRC中最显着增加的FOX基因之一)的作用,以及它在CRC转移中被解除调节的机制。方法:在两个独立的人类CRC队列中分析了FOXK2水平(队列I,n = 363;队列II,n = 390)。体外Transwell测定法和体内肺和肝转移模型用于检查CRC细胞的迁移,侵袭和转移。染色质免疫沉淀和荧光素酶报告基因分析用于测量转录因子与FOXK2启动子的结合,锌指E-box结合同源异型盒1(ZEB1)和表皮生长因子受体(EGFR)。西妥昔单抗用于治疗FOXK2介导的转移性CRC。结果:FOXK2在人CRC组织中显着上调,与更具侵略性的特征相关,预后不良。FOXK2的过表达促进了CRC的迁移,侵袭和转移,而FOXK2的下调则具有相反的作用。ZEB1和EGFR被确定为FOXK2的直接转录目标,并且是FOXK2介导的CRC转移所必需的。此外,EGF对EGFR信号的激活通过细胞外调节蛋白激酶(ERK)和核因子(NF)-κB途径增强了FOXK2的表达。EGFR单克隆抗体西妥昔单抗显着抑制FOXK2促进的CRC转移。在临床CRC组织中,FOXK2表达与p65,ZEB1和EGFR的表达呈正相关。共表达p65 / FOXK2,FOXK2 / ZEB1和FOXK2 / EGFR的CRC患者预后较差。结论:FOXK2可作为CRC的预后生物标志物。西妥昔单抗可阻断EGF-NF-κB-FOXK2-EGFR反馈回路并抑制CRC转移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号