当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Multifunctional Nanotherapy for Targeted Treatment of Colon Cancer by Simultaneously Regulating Tumor Microenvironment.
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.34377 Qixiong Zhang 1 , Fuzhong Zhang 1 , Shanshan Li 2 , Renfeng Liu 1, 3 , Taotao Jin 1, 3 , Yin Dou 1 , Zhenhua Zhou 3 , Jianxiang Zhang 1
Theranostics ( IF 12.4 ) Pub Date : 2019-01-01 , DOI: 10.7150/thno.34377 Qixiong Zhang 1 , Fuzhong Zhang 1 , Shanshan Li 2 , Renfeng Liu 1, 3 , Taotao Jin 1, 3 , Yin Dou 1 , Zhenhua Zhou 3 , Jianxiang Zhang 1
Affiliation
|
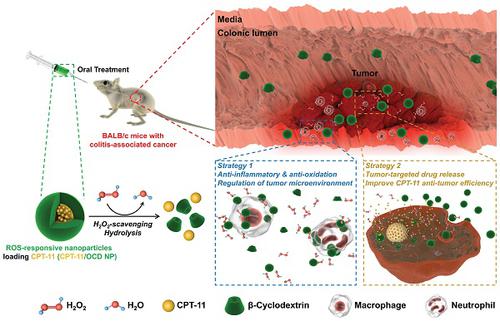
Colitis-associated colon cancer (CAC) is a widely recognized cancer, while treatment with the existing chemotherapeutic drugs affords limited clinical benefits. Herein we proposed a site-specific, combination nanotherapy strategy for targeted treatment of CAC by the oral route. Methods: A reactive oxygen species (ROS)-responsive and hydrogen peroxide-eliminating material OCD was synthesized, which was further produced into a functional nanoparticle (OCD NP). The antioxidative stress and anti-inflammatory effects of OCD NP were examined by in vitro and in vivo experiments. By packaging an anticancer drug camptothecin-11 (CPT-11) into OCD NP, a ROS-responsive nanotherapy CPT-11/OCD NP was obtained, and its antitumor activity was evaluated by both in vitro and in vivo studies. Preliminary safety studies were also performed for CPT-11/OCD NP in mice. Results: OCD NP significantly attenuated oxidative stress and inhibited inflammatory response in different cells and mice with induced colitis. CPT-11/OCD NP could selectively release drug molecules under intestinal pH conditions and at high levels of ROS. In C26 murine colon carcinoma cells, this nanotherapy showed significantly higher antitumor activity compared to free CPT-11 and a non-responsive CPT-11 nanotherapy. Correspondingly, oral delivery of CPT-11/OCD NP notably inhibited tumorigenesis and tumor growth in mice with induced CAC. By combination therapy with the nanovehicle OCD NP in the inflammatory phase, more desirable therapeutic effects were achieved. Furthermore, CPT-11/OCD NP displayed excellent safety profile for oral administration at a dose that is 87.3-fold higher than that employed in therapeutic studies. Conclusions: Anticancer nanotherapies derived from intrinsic anti-inflammatory nanocarriers are promising for targeted combination treatment of inflammation-associated tumors by simultaneously shaping pro-inflammatory microenvironment toward a relatively normal niche sensitive to chemotherapy.
中文翻译:

通过同时调节肿瘤微环境的靶向治疗结肠癌的多功能纳米疗法。
结肠炎相关的结肠癌(CAC)是一种公认的癌症,而使用现有的化疗药物进行治疗只能提供有限的临床收益。在本文中,我们提出了针对特定部位的联合纳米疗法,用于通过口服途径靶向治疗CAC。方法:合成活性氧(ROS)反应性和消除过氧化氢的物质OCD,将其进一步制备为功能纳米粒子(OCD NP)。通过体外和体内实验检查了OCD NP的抗氧化应激和抗炎作用。通过将抗癌药物喜树碱11(CPT-11)包装到OCD NP中,获得了ROS响应纳米疗法CPT-11 / OCD NP,并通过体外和体内研究评估了其抗肿瘤活性。还对小鼠中的CPT-11 / OCD NP进行了初步安全性研究。结果:OCD NP可显着减轻氧化应激并抑制不同细胞和小鼠结肠炎的炎症反应。CPT-11 / OCD NP可以在肠道pH条件和高水平ROS下选择性释放药物分子。在C26鼠结肠癌细胞中,与游离CPT-11和无反应性CPT-11纳米疗法相比,这种纳米疗法显示出显着更高的抗肿瘤活性。相应地,CPT-11 / OCD NP的口服给药显着抑制了诱发CAC的小鼠的肿瘤发生和肿瘤生长。通过与处于炎性期的纳米载体OCD NP的组合疗法,获得了更理想的治疗效果。此外,CPT-11 / OCD NP在口服治疗方面显示出出色的安全性,其剂量比治疗研究中使用的剂量高87.3倍。结论:
更新日期:2019-01-01
中文翻译:

通过同时调节肿瘤微环境的靶向治疗结肠癌的多功能纳米疗法。
结肠炎相关的结肠癌(CAC)是一种公认的癌症,而使用现有的化疗药物进行治疗只能提供有限的临床收益。在本文中,我们提出了针对特定部位的联合纳米疗法,用于通过口服途径靶向治疗CAC。方法:合成活性氧(ROS)反应性和消除过氧化氢的物质OCD,将其进一步制备为功能纳米粒子(OCD NP)。通过体外和体内实验检查了OCD NP的抗氧化应激和抗炎作用。通过将抗癌药物喜树碱11(CPT-11)包装到OCD NP中,获得了ROS响应纳米疗法CPT-11 / OCD NP,并通过体外和体内研究评估了其抗肿瘤活性。还对小鼠中的CPT-11 / OCD NP进行了初步安全性研究。结果:OCD NP可显着减轻氧化应激并抑制不同细胞和小鼠结肠炎的炎症反应。CPT-11 / OCD NP可以在肠道pH条件和高水平ROS下选择性释放药物分子。在C26鼠结肠癌细胞中,与游离CPT-11和无反应性CPT-11纳米疗法相比,这种纳米疗法显示出显着更高的抗肿瘤活性。相应地,CPT-11 / OCD NP的口服给药显着抑制了诱发CAC的小鼠的肿瘤发生和肿瘤生长。通过与处于炎性期的纳米载体OCD NP的组合疗法,获得了更理想的治疗效果。此外,CPT-11 / OCD NP在口服治疗方面显示出出色的安全性,其剂量比治疗研究中使用的剂量高87.3倍。结论:











































 京公网安备 11010802027423号
京公网安备 11010802027423号