npj Regenerative Medicine ( IF 6.4 ) Pub Date : 2019-05-13 , DOI: 10.1038/s41536-019-0073-8 Marion T Turnbull 1 , Abba C Zubair 2 , James F Meschia 3 , William D Freeman 3, 4, 5
|
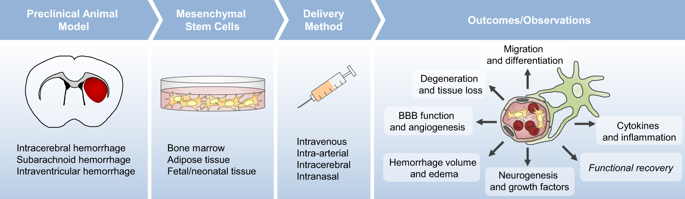
Significant progress has been made during the past few decades in stem cell therapy research for various diseases and injury states; however this has not been overwhelmingly translated into approved therapies, despite much public attention and the rise in unregulated ‘regenerative clinics’. In the last decade, preclinical research focusing on mesenchymal stem/stromal cell (MSC) therapy in experimental animal models of hemorrhagic stroke has gained momentum and has led to the development of a small number of human trials. Here we review the current studies focusing on MSC therapy for hemorrhagic stroke in an effort to summarize the status of preclinical and clinical research. Preliminary evidence indicates that MSCs are both safe and tolerable in patients, however future randomized controlled trials are required to translate the promising preclinical research into an effective therapy for hopeful patients.
中文翻译:

间充质干细胞治疗出血性中风:临床前和临床研究现状
过去几十年来,针对各种疾病和损伤状态的干细胞治疗研究取得了重大进展;然而,尽管公众广泛关注并且不受监管的“再生诊所”有所增加,但这还没有被绝大多数转化为批准的疗法。在过去的十年中,针对出血性中风实验动物模型的间充质干细胞/基质细胞(MSC)疗法的临床前研究势头强劲,并导致了少量人体试验的开展。我们回顾了当前以间充质干细胞治疗出血性脑卒中为重点的研究,以总结临床前和临床研究的现状。初步证据表明间充质干细胞对患者来说既安全又可耐受,但未来需要进行随机对照试验,将有希望的临床前研究转化为对有希望的患者的有效疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号