Metabolic Engineering ( IF 6.8 ) Pub Date : 2019-05-09 , DOI: 10.1016/j.ymben.2019.05.001 Yiqi Liu , Chenxiao Bai , Qi Liu , Qin Xu , Zhilan Qian , Qiangqiang Peng , Jiahui Yu , Mingqiang Xu , Xiangshan Zhou , Yuanxing Zhang , Menghao Cai
|
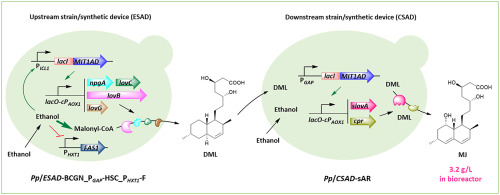
Many natural drugs use acetyl-CoA as the key biosynthetic precursor. While in eukaryotic chassis host like yeast, efficient biosynthesis of these drugs is often hampered by insufficient acetyl-CoA supply because of its compartmentalized metabolism. Reported acetyl-CoA engineering commonly modifies central carbon metabolism to pull and push acetyl-CoA into cytosol from sugars or redirects biosynthetic pathways in organelles, involving complicated metabolic engineering strategies. We constructed a new biosynthetic system based on a Crabtree-negative yeast, which grew exceptionally on ethanol and assimilated ethanol directly in cytosol to acetyl-CoA (3 steps). A glucose-repressed and ethanol-induced transcriptional signal amplification device (ESAD) with 20-fold signal increase was constructed by rewiring native transcriptional regulation circuits. This made ethanol the sole and fast-growing substrate, acetyl-CoA precursor, and strong biosynthetic pathway inducer simultaneously. The ESAD was used for biosynthesis of a commercial hypolipidemic drug intermediate, monacolin J. A strain producing dihydromonacolin L was firstly constructed and systematically engineered. We further developed a coculture system equipped with this upstream strain and a downstream strain with dihydromonacolin L–to–monacolin J module controlled by a synthetic constitutive transcriptional signal amplification device (CSAD). It produced a high monacolin J titre of 2.2 g/L on ethanol in bioreactor. Engineering glucose-supported and ethanol-repressed fatty acids biosynthesis in the upstream strain contributed more acetyl-CoA for monacolin J and improved its titre to 3.2 g/L, far surpassing other reported productions in yeasts. This study provides a new paradigm for facilitating the high-yield production of acetyl-CoA derived pharmaceuticals and value-added molecules.
中文翻译:

工程乙醇驱动的生物合成系统,可改善Crabtree阴性酵母中乙酰辅酶A衍生药物的生产
许多天然药物使用乙酰辅酶A作为关键的生物合成前体。虽然在真核底盘宿主(如酵母)中,但是由于乙酰化CoA的分区代谢,因此它们的有效生物合成通常因乙酰辅酶A的供应不足而受到阻碍。报道的乙酰辅酶A工程通常会修饰中心碳代谢,以将乙酰辅酶A从糖中拉出或推入细胞质中,或重定向细胞器中的生物合成途径,这涉及复杂的代谢工程策略。我们基于Crabtree阴性酵母构建了一个新的生物合成系统,该酵母在乙醇和同化乙醇中直接在细胞溶质中直接转化为乙酰辅酶A(3个步骤)。通过重新连接天然转录调控电路,构建了葡萄糖抑制和乙醇诱导的转录信号放大装置(ESAD),其信号增加了20倍。这使乙醇同时成为唯一且快速增长的底物,乙酰辅酶A前体和强大的生物合成途径诱导剂。ESAD用于商业合成降血脂药物中间体莫纳可林J的生物合成。首先构建并系统工程化了生产二氢莫纳可林L的菌株。我们进一步开发了配备该上游菌株和下游菌株的共培养系统,该菌株带有由合成组成型转录信号放大装置(CSAD)控制的二氢莫纳可林L至莫纳可林J模块。在生物反应器中,乙醇产生的莫纳可林J滴定度高,为2.2 g / L。上游菌株中工程葡萄糖支持和乙醇抑制的脂肪酸生物合成为莫纳可林J贡献了更多的乙酰辅酶A,并将其效价提高至3.2 g / L,远远超过其他报道的酵母产量。这项研究为促进乙酰辅酶A衍生药物和增值分子的高产量生产提供了新的范例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号