Nature Reviews Chemistry ( IF 38.1 ) Pub Date : 2019-05-07 , DOI: 10.1038/s41570-019-0099-x Huw M. L. Davies , Kuangbiao Liao
|
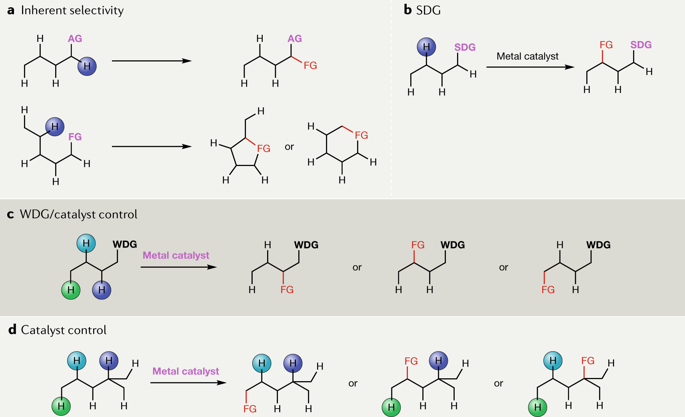
C–H functionalization has become widely recognized as an exciting new strategy for the synthesis of complex molecular targets. Instead of relying on functional groups as the controlling elements of how molecules are assembled, this strategy offers an altogether different logic for organic synthesis. For this type of strategy to be successful, reagents and catalysts need to be developed that generate intermediates that are sufficiently reactive to functionalize C–H bonds but are still capable of distinguishing between the many different C–H bonds and other functional groups present in a molecule. The most well-established approaches have tended to use substrates that inherently have a favoured site for C–H functionalization or rely on intramolecular reactions to control where the reaction will occur. A challenging but potentially more versatile approach would be to use catalysts to control the site selectivity without requiring the influence of any directing group. One example that is capable of achieving such transformations is the C–H insertion chemistry of transient metal carbenes. Dirhodium tetracarboxylates have been shown to be especially effective catalysts for these reactions. This Review highlights the development of these dirhodium catalysts and illustrates their effectiveness to control both site-selective and stereoselective C–H functionalization of a wide variety of substrates.
中文翻译:

四羧酸二铱酯作为选择性分子间C–H官能化的催化剂
C–H功能化已被广泛认为是合成复杂分子靶标的令人兴奋的新策略。该策略不再依赖官能团作为分子组装方式的控制元素,而是为有机合成提供了完全不同的逻辑。为使这种策略成功,需要开发出能够产生足够反应性以官能化C–H键但仍能够区分许多不同的C–H键和其他官能团的中间体的试剂和催化剂。分子。最完善的方法倾向于使用固有具有CH功能官能化位点的底物,或依靠分子内反应控制反应发生的位置。一个有挑战性但可能更通用的方法是使用催化剂来控制位点选择性,而无需任何指导基团的影响。能够实现这种转变的一个例子是瞬态金属卡宾的C–H插入化学。已经证明四羧酸二铱酯是用于这些反应的特别有效的催化剂。这篇综述重点介绍了这些催化剂的发展,并说明了它们在控制多种底物的位点选择性和立体选择性CH官能化方面的有效性。已经证明四羧酸二铱酯是用于这些反应的特别有效的催化剂。这篇综述重点介绍了这些催化剂的发展,并说明了它们在控制多种底物的位点选择性和立体选择性CH官能化方面的有效性。已经证明四羧酸二铱酯是用于这些反应的特别有效的催化剂。这篇综述重点介绍了这些催化剂的开发,并说明了它们在控制多种底物的位点选择性和立体选择性CH官能化方面的有效性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号