当前位置:
X-MOL 学术
›
J. Food Drug Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The biosimilar pathway in the USA: An analysis of the innovator company and biosimilar company perspectives and beyond
Journal of Food and Drug Analysis ( IF 2.6 ) Pub Date : 2019-07-01 , DOI: 10.1016/j.jfda.2019.03.003 Lin-Chau Chang
Journal of Food and Drug Analysis ( IF 2.6 ) Pub Date : 2019-07-01 , DOI: 10.1016/j.jfda.2019.03.003 Lin-Chau Chang
|
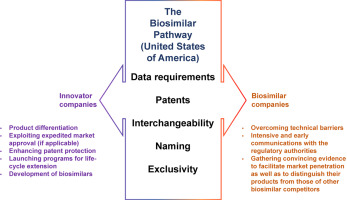
In order to improve access to costly biological treatments, a biosimilar pathway in the United States of America (USA) was enacted under the Biologics Price Competition and Innovation Act (BPCI Act) of 2009. The aim of the present study was to investigate how the health policy, the establishment of the biosimilar pathway, influenced related companies by studying their respective perspectives and strategies revealed in literatures and publicly available resources. Perspectives of companies reveal the points of concern for the biosimilar pathway, such as data requirements, patents, interchangeability, naming, and exclusivity. Innovator companies may utilize expedited programs for serious conditions, enhance patent protection, launch programs for life-cycle extension, and develop biosimilars as well. The biosimilar companies overcoming technical barriers might need to gather convincing evidence to facilitate market penetration as well as to distinguish their products from those of other biosimilar competitors. More challenges are expected for innovator companies if international harmonization takes place, which might be worth further investigation.
中文翻译:

美国的生物仿制药路径:对创新公司和生物仿制药公司观点及其他方面的分析
为了改善获得昂贵的生物治疗的机会,美国 (USA) 根据 2009 年的《生物制品价格竞争和创新法案》(BPCI Act) 制定了一条生物仿制药途径。本研究的目的是调查卫生政策、生物仿制药途径的建立通过研究文献和公开资源中揭示的各自观点和策略来影响相关公司。公司的观点揭示了生物仿制药途径的关注点,例如数据要求、专利、可互换性、命名和排他性。创新公司可以针对严重疾病利用加速计划、加强专利保护、启动生命周期延长计划以及开发生物仿制药。克服技术壁垒的生物仿制药公司可能需要收集令人信服的证据以促进市场渗透,并将其产品与其他生物仿制药竞争对手的产品区分开来。如果发生国际协调,创新公司将面临更多挑战,这可能值得进一步研究。
更新日期:2019-07-01
中文翻译:

美国的生物仿制药路径:对创新公司和生物仿制药公司观点及其他方面的分析
为了改善获得昂贵的生物治疗的机会,美国 (USA) 根据 2009 年的《生物制品价格竞争和创新法案》(BPCI Act) 制定了一条生物仿制药途径。本研究的目的是调查卫生政策、生物仿制药途径的建立通过研究文献和公开资源中揭示的各自观点和策略来影响相关公司。公司的观点揭示了生物仿制药途径的关注点,例如数据要求、专利、可互换性、命名和排他性。创新公司可以针对严重疾病利用加速计划、加强专利保护、启动生命周期延长计划以及开发生物仿制药。克服技术壁垒的生物仿制药公司可能需要收集令人信服的证据以促进市场渗透,并将其产品与其他生物仿制药竞争对手的产品区分开来。如果发生国际协调,创新公司将面临更多挑战,这可能值得进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号