当前位置:
X-MOL 学术
›
npj Vaccines
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent advances in the production of recombinant glycoconjugate vaccines.
npj Vaccines ( IF 6.9 ) Pub Date : 2019-05-01 , DOI: 10.1038/s41541-019-0110-z Emily Kay 1 , Jon Cuccui 1 , Brendan W Wren 1
npj Vaccines ( IF 6.9 ) Pub Date : 2019-05-01 , DOI: 10.1038/s41541-019-0110-z Emily Kay 1 , Jon Cuccui 1 , Brendan W Wren 1
Affiliation
|
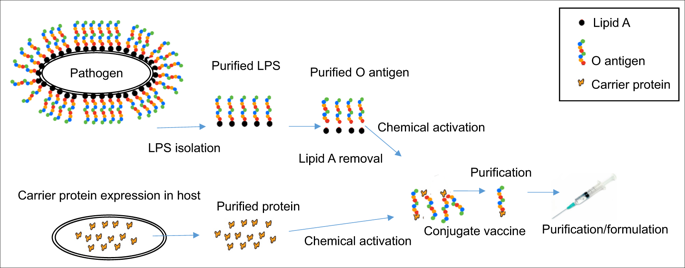
Glycoconjugate vaccines against bacteria are one of the success stories of modern medicine and have led to a significant reduction in the global occurrence of bacterial meningitis and pneumonia. Glycoconjugate vaccines are produced by covalently linking a bacterial polysaccharide (usually capsule, or more recently O-antigen), to a carrier protein. Given the success of glycoconjugate vaccines, it is surprising that to date only vaccines against Haemophilus influenzae type b, Neisseria meningitis and Streptococcus pneumoniae have been fully licenced. This is set to change through the glycoengineering of recombinant vaccines in bacteria, such as Escherichia coli, that act as mini factories for the production of an inexhaustible and renewable supply of pure vaccine product. The recombinant process, termed Protein Glycan Coupling Technology (PGCT) or bioconjugation, offers a low-cost option for the production of pure glycoconjugate vaccines, with the in-built flexibility of adding different glycan/protein combinations for custom made vaccines. Numerous vaccine candidates have now been made using PGCT, which include those improving existing licenced vaccines (e.g., pneumococcal), entirely new vaccines for both Gram-positive and Gram-negative bacteria, and (because of the low production costs) veterinary pathogens. Given the continued threat of antimicrobial resistance and the potential peril of bioterrorist agents, the production of new glycoconjugate vaccines against old and new bacterial foes is particularly timely. In this review, we will outline the component parts of bacterial PGCT, including recent advances, the advantages and limitations of the technology, and future applications and perspectives.
中文翻译:

重组糖结合疫苗生产的最新进展。
针对细菌的糖缀合物疫苗是现代医学的成功案例之一,并导致全球细菌性脑膜炎和肺炎的发生显着减少。糖结合疫苗是通过将细菌多糖(通常是荚膜,或最近的 O 抗原)与载体蛋白共价连接而生产的。鉴于糖结合疫苗的成功,令人惊讶的是,迄今为止只有针对 b 型流感嗜血杆菌、脑膜炎奈瑟菌和肺炎链球菌的疫苗获得了完全许可。这将通过细菌重组疫苗的糖工程来改变,例如大肠杆菌,这些疫苗充当微型工厂,用于生产取之不尽、可再生的纯疫苗产品供应。重组过程,称为蛋白聚糖偶联技术 (PGCT) 或生物偶联技术,为生产纯糖偶联疫苗提供了一种低成本的选择,具有为定制疫苗添加不同聚糖/蛋白质组合的内在灵活性。现在已经使用 PGCT 制备了许多候选疫苗,其中包括那些改进现有许可疫苗(例如肺炎球菌)的疫苗、针对革兰氏阳性和革兰氏阴性细菌的全新疫苗,以及(因为生产成本低)兽用病原体。鉴于抗菌素耐药性的持续威胁和生物恐怖剂的潜在危险,针对新旧细菌敌人的新糖结合疫苗的生产特别及时。在这篇综述中,我们将概述细菌 PGCT 的组成部分,包括最近的进展,
更新日期:2019-11-18
中文翻译:

重组糖结合疫苗生产的最新进展。
针对细菌的糖缀合物疫苗是现代医学的成功案例之一,并导致全球细菌性脑膜炎和肺炎的发生显着减少。糖结合疫苗是通过将细菌多糖(通常是荚膜,或最近的 O 抗原)与载体蛋白共价连接而生产的。鉴于糖结合疫苗的成功,令人惊讶的是,迄今为止只有针对 b 型流感嗜血杆菌、脑膜炎奈瑟菌和肺炎链球菌的疫苗获得了完全许可。这将通过细菌重组疫苗的糖工程来改变,例如大肠杆菌,这些疫苗充当微型工厂,用于生产取之不尽、可再生的纯疫苗产品供应。重组过程,称为蛋白聚糖偶联技术 (PGCT) 或生物偶联技术,为生产纯糖偶联疫苗提供了一种低成本的选择,具有为定制疫苗添加不同聚糖/蛋白质组合的内在灵活性。现在已经使用 PGCT 制备了许多候选疫苗,其中包括那些改进现有许可疫苗(例如肺炎球菌)的疫苗、针对革兰氏阳性和革兰氏阴性细菌的全新疫苗,以及(因为生产成本低)兽用病原体。鉴于抗菌素耐药性的持续威胁和生物恐怖剂的潜在危险,针对新旧细菌敌人的新糖结合疫苗的生产特别及时。在这篇综述中,我们将概述细菌 PGCT 的组成部分,包括最近的进展,











































 京公网安备 11010802027423号
京公网安备 11010802027423号