当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Equilibria and Phase Diagrams for the Aqueous Ternary System Containing Sodium, Sulfate, and Metaborate Ions at 288.15 and 308.15 K and 101.325 kPa
Journal of Chemical & Engineering Data ( IF 2.1 ) Pub Date : 2019-05-01 , DOI: 10.1021/acs.jced.9b00177 Shangqing Chen , Wanjing Cui , Jiayin Hu , Yafei Guo , Tianlong Deng
Journal of Chemical & Engineering Data ( IF 2.1 ) Pub Date : 2019-05-01 , DOI: 10.1021/acs.jced.9b00177 Shangqing Chen , Wanjing Cui , Jiayin Hu , Yafei Guo , Tianlong Deng
|
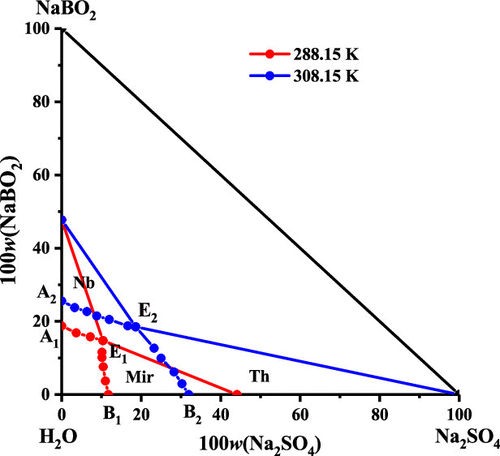
Solubilities and physicochemical properties (density and refractive index) for the ternary system (Na+//SO42–, BO2–-H2O) at (288.15 and 308.15) K and 101.325 kPa were studied by the isothermal dissolution equilibrium method. Phase diagrams and diagrams of the physicochemical properties (density and refractive index) were plotted against the concentration of sodium sulfate. For both temperatures, the phase diagrams contained one invariant point, two univariant curves, and two crystallization regions corresponding to sodium metaborate tetrahydrate (NaBO2·4H2O, Nb) and mirabilite (Na2SO4·10H2O, Mir) at 288.15 K, and Nb and thenardite (Na2SO4, Th) at 308.15 K, respectively. The area of the crystallization region of sodium metaborate tetrahydrate was enlarged with the increase of temperature, and the solid phase of mirabilite transformed into thenardite when the temperature increased from 288.15 to 308.15 K. The physiochemical properties including density and refractive index of the system at (288.15 and 308.15) K were similarly increased first and decreased later with the increase of sodium sulfate concentration in the equilibrium solution. The experimental data of the density and refractive index were correlated using the empirical equations, and the calculated and experimental values agreed well.
中文翻译:

包含钠离子,硫酸根离子和偏硼酸根离子的水性三元体系在288.15和308.15 K和101.325 kPa的相平衡和相图
通过等温溶出平衡法研究了三元体系(Na + // SO 4 2–,BO 2 – -H 2 O)在(288.15和308.15)K和101.325 kPa时的溶解度和理化性质(密度和折射率)。相对于硫酸钠的浓度绘制了相图和理化性质(密度和折射率)图。对于两种温度,相图均包含一个不变点,两个单变量曲线和两个结晶区,分别对应于偏硼酸钠四水合物(NaBO 2 ·4H 2 O,Nb)和芒硝(Na 2 SO 4 ·10H 2)O,Mir)在288.15 K,Nb和芒硝(Na 2 SO 4,Th)在308.15K。随着温度的升高,偏硼酸钠四水合物的结晶区域面积增大,当温度从288.15 K升高到308.15 K时,芒硝的固相转变为芒硝。 288.15和308.15)K随平衡溶液中硫酸钠浓度的增加先增加后减少。使用经验公式将密度和折射率的实验数据进行关联,计算值和实验值吻合得很好。
更新日期:2019-05-24
中文翻译:

包含钠离子,硫酸根离子和偏硼酸根离子的水性三元体系在288.15和308.15 K和101.325 kPa的相平衡和相图
通过等温溶出平衡法研究了三元体系(Na + // SO 4 2–,BO 2 – -H 2 O)在(288.15和308.15)K和101.325 kPa时的溶解度和理化性质(密度和折射率)。相对于硫酸钠的浓度绘制了相图和理化性质(密度和折射率)图。对于两种温度,相图均包含一个不变点,两个单变量曲线和两个结晶区,分别对应于偏硼酸钠四水合物(NaBO 2 ·4H 2 O,Nb)和芒硝(Na 2 SO 4 ·10H 2)O,Mir)在288.15 K,Nb和芒硝(Na 2 SO 4,Th)在308.15K。随着温度的升高,偏硼酸钠四水合物的结晶区域面积增大,当温度从288.15 K升高到308.15 K时,芒硝的固相转变为芒硝。 288.15和308.15)K随平衡溶液中硫酸钠浓度的增加先增加后减少。使用经验公式将密度和折射率的实验数据进行关联,计算值和实验值吻合得很好。























































 京公网安备 11010802027423号
京公网安备 11010802027423号