Prostate Cancer and Prostatic Diseases ( IF 5.1 ) Pub Date : 2019-04-29 , DOI: 10.1038/s41391-019-0151-4 Jose Mauricio Mota 1 , Andrew J Armstrong 2, 3 , Steven M Larson 4, 5 , Josef J Fox 5, 6 , Michael J Morris 1, 7
|
Background
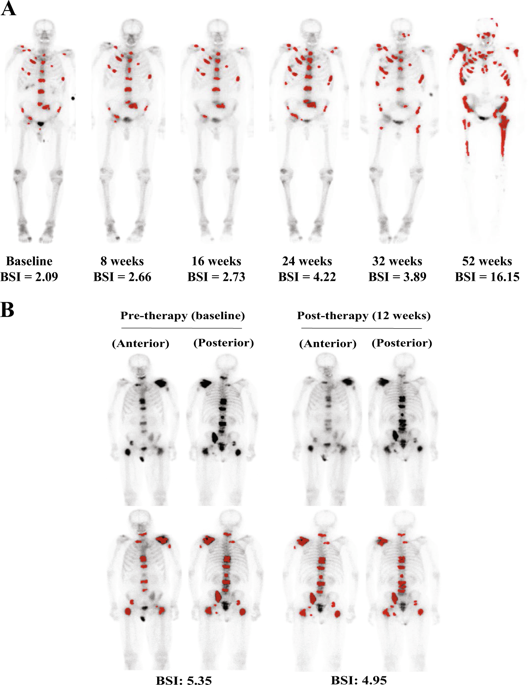
Up to 90% of men with metastatic castration-resistant prostate cancer (mCRPC) will have a distribution of disease that includes bone metastases demonstrated on a Technetium-99m (99mTc-MDP) bone scan. The Prostate Cancer Working Group 2 and 3 Consensus Criteria standardized the criteria for assessing progression based on the development of new lesions. These criteria have been recognized by regulatory authorities for drug approval. The bone scan index (BSI) is a method to quantitatively measure the burden of bony disease, and can assess both disease progression and regression. The automated BSI (aBSI) is a method of computer analysis to assess BSI, and is being qualified as a clinical trials endpoint.
Methods
Manual searching was used to identify the literature on BSI and aBSI. We summarize the most relevant aspects of the retrospective and prospective studies evaluating aBSI measurements, and provide a critical discussion on the potential advantages and caveats of aBSI.
Results
The development of neural artificial networks (EXINI boneBSI) to automatically determine the BSI reduces the turnaround time for assessing BSI with high reproducibility and accuracy. Several studies showed that the concordance between aBSI and BSI, as well as the interobserver concordance of aBSI, was >0.95. In a phase 3 assessment of aBSI, a doubling value increased the risk of death in 20%, pre-treatment aBSI values independently correlated with overall survival (OS) and time to symptomatic progression. Retrospective studies suggest that a decrease in aBSI after treatment may correlate with higher survival when compared with increasing aBSI.
Conclusions
aBSI provides a quantitative measurement that is feasible, reproducible, and in analyses to date correlates with OS and symptomatic progression. These findings support the aBSI to risk-stratify men with mCRPC for clinical trial enrollment. Future studies quantifying aBSI change over time as an intermediate endpoint for evaluating new systemic therapies are needed.
中文翻译:

测量不可测量:自动骨扫描指数作为前列腺癌临床试验中的定量终点。
背景
高达 90% 的转移性去势抵抗性前列腺癌 (mCRPC) 男性的疾病分布包括锝 99m ( 99m Tc-MDP) 骨扫描显示的骨转移。前列腺癌工作组 2 和 3 共识标准标准化了基于新病变发展评估进展的标准。这些标准已被监管机构认可用于药物批准。骨扫描指数 (BSI) 是一种定量测量骨疾病负担的方法,可以评估疾病进展和消退。自动化 BSI (aBSI) 是一种评估 BSI 的计算机分析方法,并被认定为临床试验终点。
方法
人工检索用于识别关于 BSI 和 aBSI 的文献。我们总结了评估 aBSI 测量的回顾性和前瞻性研究中最相关的方面,并对 aBSI 的潜在优势和注意事项进行了批判性讨论。
结果
用于自动确定 BSI 的神经人工网络 (EXINI boneBSI) 的开发缩短了以高重现性和准确性评估 BSI 的周转时间。几项研究表明,aBSI 和 BSI 之间的一致性以及 aBSI 的观察者间一致性> 0.95。在 aBSI 的 3 期评估中,加倍值增加了 20% 的死亡风险,治疗前 aBSI 值与总生存期 (OS) 和症状进展时间独立相关。回顾性研究表明,与增加的 aBSI 相比,治疗后 aBSI 的降低可能与更高的生存率相关。
结论
aBSI 提供了一种可行的、可重复的定量测量,并且在迄今为止的分析中与 OS 和症状进展相关。这些发现支持 aBSI 对患有 mCRPC 的男性进行风险分层以进行临床试验登记。未来需要量化 aBSI 随时间变化的研究,作为评估新全身疗法的中间终点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号