Bone Research ( IF 14.3 ) Pub Date : 2019-04-25 , DOI: 10.1038/s41413-019-0049-8 Nathalie Bock 1, 2, 3 , Ali Shokoohmand 1, 2, 3 , Thomas Kryza 1, 2 , Joan Röhl 1, 2 , Jonelle Meijer 1, 2, 3 , Phong A Tran 3, 4 , Colleen C Nelson 1, 2 , Judith A Clements 1, 2 , Dietmar W Hutmacher 1, 2, 3, 4, 5
|
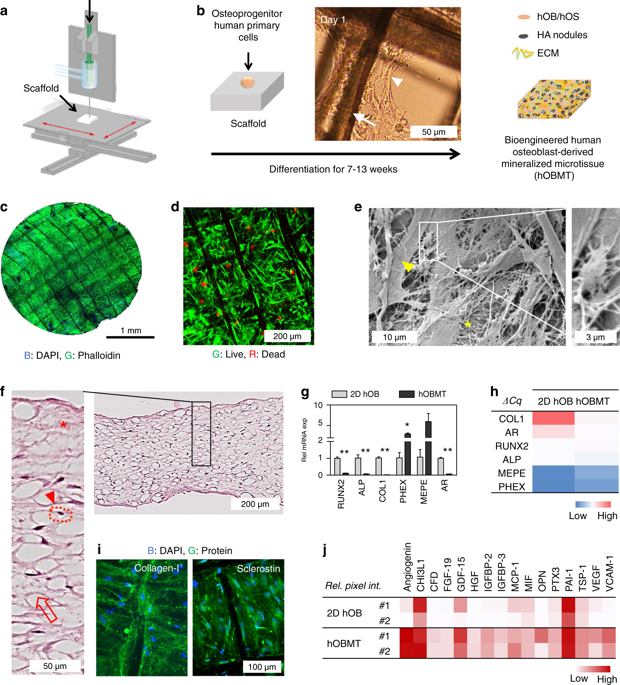
While stromal interactions are essential in cancer adaptation to hormonal therapies, the effects of bone stroma and androgen deprivation on cancer progression in bone are poorly understood. Here, we tissue-engineered and validated an in vitro microtissue model of osteoblastic bone metastases, and used it to study the effects of androgen deprivation in this microenvironment. The model was established by culturing primary human osteoprogenitor cells on melt electrowritten polymer scaffolds, leading to a mineralized osteoblast-derived microtissue containing, in a 3D setting, viable osteoblastic cells, osteocytic cells, and appropriate expression of osteoblast/osteocyte-derived mRNA and proteins, and mineral content. Direct co-culture of androgen receptor-dependent/independent cell lines (LNCaP, C4-2B, and PC3) led cancer cells to display functional and molecular features as observed in vivo. Co-cultured cancer cells showed increased affinity to the microtissues, as a function of their bone metastatic potential. Co-cultures led to alkaline phosphatase and collagen-I upregulation and sclerostin downregulation, consistent with the clinical marker profile of osteoblastic bone metastases. LNCaP showed a significant adaptive response under androgen deprivation in the microtissues, with the notable appearance of neuroendocrine transdifferentiation features and increased expression of related markers (dopa decarboxylase, enolase 2). Androgen deprivation affected the biology of the metastatic microenvironment with stronger upregulation of androgen receptor, alkaline phosphatase, and dopa decarboxylase, as seen in the transition towards resistance. The unique microtissues engineered here represent a substantial asset to determine the involvement of the human bone microenvironment in prostate cancer progression and response to a therapeutic context in this microenvironment.
中文翻译:

工程化成骨细胞转移以描绘骨转移微环境中雄激素剥夺的前列腺癌的适应性反应
虽然基质相互作用对于癌症适应激素治疗至关重要,但骨基质和雄激素剥夺对骨癌症进展的影响却知之甚少。在这里,我们组织工程并验证了成骨细胞骨转移的体外微组织模型,并用它来研究雄激素剥夺在此微环境中的影响。该模型是通过在熔融电写聚合物支架上培养原代人骨祖细胞而建立的,从而产生矿化的成骨细胞衍生的微组织,在 3D 环境中含有存活的成骨细胞、骨细胞以及成骨细胞/骨细胞衍生的 mRNA 和蛋白质的适当表达和矿物质含量。雄激素受体依赖性/非依赖性细胞系(LNCaP、C4-2B 和 PC3)的直接共培养使癌细胞表现出体内观察到的功能和分子特征。共培养的癌细胞表现出与微组织的亲和力增加,这是其骨转移潜力的函数。共培养导致碱性磷酸酶和胶原蛋白 I 上调以及硬化素下调,这与成骨细胞骨转移的临床标志物特征一致。LNCaP在雄激素剥夺下微组织中表现出显着的适应性反应,神经内分泌转分化特征显着出现,相关标记物(多巴脱羧酶、烯醇化酶2)表达增加。雄激素剥夺影响了转移微环境的生物学,雄激素受体、碱性磷酸酶和多巴脱羧酶的上调更强,如向耐药性的转变所示。这里设计的独特微组织代表了确定人骨微环境在前列腺癌进展中的参与以及对该微环境中治疗环境的反应的重要资产。











































 京公网安备 11010802027423号
京公网安备 11010802027423号