Catalysis Today ( IF 5.2 ) Pub Date : 2019-04-19 , DOI: 10.1016/j.cattod.2019.04.066 Sardar Ali , Mahmoud M. Khader , Mohammed J. Almarri , Ahmed G. Abdelmoneim
|
Development of a highly efficient and coke-resistant, nickel based nano-catalyst in the carbon dioxide reformation of methane is reported. The alumina supported Ni-based catalyst with a metal loading of 5wt% was prepared via the solution combustion synthesis (SCS) method as well as the conventional wetness impregnation method. The synthesized catalysts were thoroughly characterized by a combination of analytical techniques including high-resolution electron microscopy (HRTEM-SAED), X-ray diffraction (XRD), nitrogen physisorption (BET surface area), X-ray photoelectron spectroscopy (XPS), temperature programmed reduction (H2-TPR) and temperature programmed oxidation (TPO).
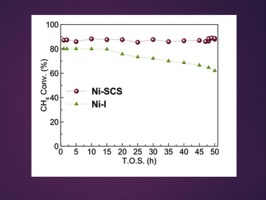
Compared to the conventional nickel-impregnated (Ni-I) catalyst, the Ni-SCS nano-catalyst was superior in activity and stability during dry reformation of methane. Ni-SCS catalyst exhibited higher percentage conversions of methane and carbon dioxide. The percentage yields of hydrogen and carbon monoxide over Ni-SCS catalyst were also significantly higher. During the investigated period on stream for 50 h, the Ni-I catalyst deactivated severely, by contrast the Ni-SCS stayed active. It was clear from the results of elemental carbon analysis and TPO that deactivation of the Ni-I catalyst was due to severe carbon deposition, whereas the Ni-SCS catalyst exhibited minor carbon deposition. These differences in the catalytic activities and stabilities between the Ni-I and Ni-SCS catalysts were attributed to the difference in their physicochemical properties and chemical structure, as obvious from the results of the above mentioned analysis techniques.
The XRD and XPS analysis revealed that the Ni-SCS nanocatalyst resulted in the formation of uniformly distributed nickel aluminates (NiAl2O4) nano-crystallite spinels together with nickel oxide. The results of H2-TPR analysis clearly distinguished between NiO and NiAl2O4. H2-TPR affirmed the formation of NiAl2O4 and NiO species on the SCS nanocatalyst but only NiO within the impregnation catalyst. In this regards the exceptionally high catalytic activity and stability of Ni-SCS nanocatalyst during dry reformation was attributed to the presence of NiAl2O4 nano-crystallites structures. On the other hand, the presence of weakly associated NiO species on the Ni-I catalyst was responsible for decaying its activity due to carbon formation during the dry reformation of methane.
中文翻译:

用于甲烷干重整的镍基纳米催化剂
据报道,在甲烷的二氧化碳重整中开发了一种高效且耐焦炭的镍基纳米催化剂。通过固溶燃烧合成(SCS)方法以及常规的湿法浸渍方法制备了具有5wt%的金属负载的氧化铝负载的Ni基催化剂。合成的催化剂通过包括高分辨率电子显微镜(HRTEM-SAED),X射线衍射(XRD),氮物理吸附(BET表面积),X射线光电子能谱(XPS),温度等分析技术的组合进行了全面表征。程序还原(H 2 -TPR)和程序氧化(TPO)。
与常规的镍浸渍(Ni-I)催化剂相比,Ni-SCS纳米催化剂在甲烷干重整过程中具有出色的活性和稳定性。Ni-SCS催化剂显示出较高的甲烷和二氧化碳转化率。Ni-SCS催化剂上氢气和一氧化碳的百分收率也明显更高。在研究期间,在运行50小时期间,Ni-I催化剂严重失活,相比之下,Ni-SCS保持活性。从元素碳分析和TPO的结果可以清楚地看出,Ni-I催化剂的失活是由于严重的碳沉积,而Ni-SCS催化剂表现出较小的碳沉积。
XRD和XPS分析表明,Ni-SCS纳米催化剂与氧化镍一起形成了均匀分布的铝酸镍(NiAl 2 O 4)纳米微晶尖晶石。H 2 -TPR分析的结果清楚地区分了NiO和NiAl 2 O 4。H 2 -TPR证实了SCS纳米催化剂上NiAl 2 O 4和NiO物种的形成,但浸渍催化剂中只有NiO的形成。在这方面,Ni-SCS纳米催化剂在干重整过程中异常高的催化活性和稳定性归因于NiAl 2 O 4的存在纳米微晶结构。另一方面,Ni-I催化剂上弱缔合的NiO物种的存在是由于甲烷在干法重整过程中形成碳而导致其活性下降的原因。









































 京公网安备 11010802027423号
京公网安备 11010802027423号