当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N-Heterocyclic carbene-catalyzed diastereoselective synthesis of sulfenylated indanes via sulfa-Michael–Michael (aldol) cascade reactions
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2019-04-12 , DOI: 10.1039/c9ob00210c Ze-Nan Feng 1, 2, 3 , Jin-Yun Luo 1, 2, 3 , Yang Zhang 1, 2, 3 , Guang-Fen Du 1, 2, 3 , Lin He 1, 2, 3
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2019-04-12 , DOI: 10.1039/c9ob00210c Ze-Nan Feng 1, 2, 3 , Jin-Yun Luo 1, 2, 3 , Yang Zhang 1, 2, 3 , Guang-Fen Du 1, 2, 3 , Lin He 1, 2, 3
Affiliation
|
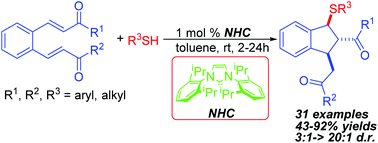
N-Heterocyclic carbene (NHC)-catalyzed diastereoselective synthesis of multisubstituted sulfenylated indanes has been developed. In the presence of 1 mol% NHC, various thiols underwent the sulfa-Michael–Michael cascade reaction with benzenedi(enones) efficiently to form the carbon–sulfur bond and construct sulfenylated indanes in good to excellent yields with high diastereoselectivity. In addition, the NHC-catalyzed sulfa-Michael-aldol cascade reaction between o-formyl chalcone and thiols has also been demonstrated to afford sulfenylated indanes with a free hydroxyl group in moderate yields and good diastereoselectivity.
中文翻译:

N-杂环卡宾通过磺胺-迈克尔-迈克尔(醛醇)级联反应催化亚砜基茚满的非对映选择性合成
已经开发了N-杂环卡宾(NHC)催化的多取代亚硫基化茚满的非对映选择性合成。在1 mol%的NHC存在下,各种硫醇与苯二(烯)进行磺胺-迈克尔-迈克尔级联反应,有效地形成碳-硫键,并以良好的非对映选择性高至优异的产率构建了亚磺酰化的茚满。另外,还已经证明邻甲酰基查尔酮和硫醇之间的NHC催化的磺胺-迈克尔-醛醇级联反应可以中等产率和良好的非对映选择性提供带有游离羟基的亚磺酰化茚满。
更新日期:2019-05-16
中文翻译:

N-杂环卡宾通过磺胺-迈克尔-迈克尔(醛醇)级联反应催化亚砜基茚满的非对映选择性合成
已经开发了N-杂环卡宾(NHC)催化的多取代亚硫基化茚满的非对映选择性合成。在1 mol%的NHC存在下,各种硫醇与苯二(烯)进行磺胺-迈克尔-迈克尔级联反应,有效地形成碳-硫键,并以良好的非对映选择性高至优异的产率构建了亚磺酰化的茚满。另外,还已经证明邻甲酰基查尔酮和硫醇之间的NHC催化的磺胺-迈克尔-醛醇级联反应可以中等产率和良好的非对映选择性提供带有游离羟基的亚磺酰化茚满。



























 京公网安备 11010802027423号
京公网安备 11010802027423号