Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of a peptide derived from a Bothrops moojeni metalloprotease with in vitro inhibitory action on the Plasmodium falciparum purine nucleoside phosphorylase enzyme (PfPNP).
Biochimie ( IF 3.3 ) Pub Date : 2019-04-09 , DOI: 10.1016/j.biochi.2019.04.009 Gracianny Gomes Martins 1 , Rudson de Jesus Holanda 2 , Jorge Alfonso 2 , Ana Fidelina Gómez Garay 2 , Ana Paula de Azevedo Dos Santos 3 , Anderson Maciel de Lima 4 , Aleff Ferreira Francisco 4 , Carolina Bioni Garcia Teles 5 , Fernando Berton Zanchi 2 , Andreimar Martins Soares 1
Biochimie ( IF 3.3 ) Pub Date : 2019-04-09 , DOI: 10.1016/j.biochi.2019.04.009 Gracianny Gomes Martins 1 , Rudson de Jesus Holanda 2 , Jorge Alfonso 2 , Ana Fidelina Gómez Garay 2 , Ana Paula de Azevedo Dos Santos 3 , Anderson Maciel de Lima 4 , Aleff Ferreira Francisco 4 , Carolina Bioni Garcia Teles 5 , Fernando Berton Zanchi 2 , Andreimar Martins Soares 1
Affiliation
|
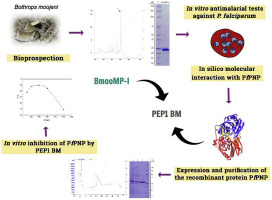
There is a growing need for research on new antimalarial agents against Plasmodium falciparum infection, especially in regards to planning molecular architecture for specific molecular targets of the parasite. Thus, a metalloprotease from Bothrops moojeni, known as BmooMPα-I, was explored in this study, through in silico assays, aiming at the development of a peptide generated from this molecule with potential inhibitory action on PfPNP, an enzyme necessary for the survival of the parasite. In order to isolate BmooMPα-I, cation exchange and reverse phase chromatographies were performed, followed by in vitro assays of antiparasitic activity against the W2 strain of P. falciparum. The interactions between BmooMPα-I and PfPNP were evaluated via docking, and the resulting peptide, described as Pep1 BM, was selected according to the BmooMPα-I region demonstrating the best interaction score with the target of interest. The values for the specific activities of the PfPNP reaction were measured using the inorganic phosphate substrate and MESG. The fraction corresponding to BmooMPα-I was identified as fraction 4 in the cation exchange chromatography step, due to proteolytic activity on casein and the presence of a major band at ≅ 23 kDa. BmooMPα-I was able to inhibit in vitro growth of W2 P. falciparum, with an IC50 value of 16.14 μg/mL. Virtual screening with Pep1 BM demonstrated two PfPNP target binding regions, with ΔG values at the interaction interface of -10.75 kcal/mol and -11.74 kcal/mol. A significant reduction in the enzymatic activity of PfPNP was observed in the presence of Pep 1 BM when compared to the assay in the absence of this possible inhibitor. BmooMPα-I showed activity in vitro against W2 P. falciparum. By means of in silico techniques, the Pep 1 BM was identified as having potential binding affinity to the catalytic site of PfPNP and of inhibiting its catalytic activity in vitro.
中文翻译:

鉴定具有对恶性疟原虫嘌呤核苷磷酸化酶(PfPNP)具有体外抑制作用的Bothrops moojeni金属蛋白酶的肽。
越来越需要研究针对恶性疟原虫感染的新型抗疟药,尤其是在针对寄生虫的特定分子靶标规划分子结构方面。因此,在这项研究中,通过计算机分析检测了来自Bothrops moojeni的金属蛋白酶,称为BmooMPα-I,旨在开发由该分子产生的对PfPNP具有潜在抑制作用的肽,PfPNP是维持PfPNP存活的酶。寄生虫。为了分离BmooMPα-1,进行了阳离子交换和反相色谱,然后体外分析了针对恶性疟原虫W2株的抗寄生虫活性。通过对接评估了BmooMPα-I与PfPNP之间的相互作用,得到的肽称为Pep1 BM,根据BmooMPα-I区域进行选择,BmooMPα-I区域显示与目标物的最佳相互作用得分。使用无机磷酸盐底物和MESG测量PfPNP反应的比活值。由于对酪蛋白的蛋白水解活性和在约23 kDa处存在一个主要谱带,因此与BmooMPα-1对应的馏分在阳离子交换色谱步骤中被鉴定为馏分4。BmooMPα-1能够抑制W2恶性疟原虫的体外生长,IC50值为16.14μg/ mL。用Pep1 BM进行的虚拟筛选显示了两个PfPNP靶标结合区域,在相互作用界面处的ΔG值分别为-10.75 kcal / mol和-11.74 kcal / mol。与在不存在这种可能的抑制剂的情况下进行的测定相比,在存在Pep 1 BM的情况下观察到PfPNP的酶活性显着降低。BmooMPα-I在体外显示出抗W2恶性疟原虫的活性。通过计算机技术,Pep 1 BM被鉴定为对PfPNP的催化位点具有潜在的结合亲和力,并在体外抑制了其催化活性。
更新日期:2019-04-09
中文翻译:

鉴定具有对恶性疟原虫嘌呤核苷磷酸化酶(PfPNP)具有体外抑制作用的Bothrops moojeni金属蛋白酶的肽。
越来越需要研究针对恶性疟原虫感染的新型抗疟药,尤其是在针对寄生虫的特定分子靶标规划分子结构方面。因此,在这项研究中,通过计算机分析检测了来自Bothrops moojeni的金属蛋白酶,称为BmooMPα-I,旨在开发由该分子产生的对PfPNP具有潜在抑制作用的肽,PfPNP是维持PfPNP存活的酶。寄生虫。为了分离BmooMPα-1,进行了阳离子交换和反相色谱,然后体外分析了针对恶性疟原虫W2株的抗寄生虫活性。通过对接评估了BmooMPα-I与PfPNP之间的相互作用,得到的肽称为Pep1 BM,根据BmooMPα-I区域进行选择,BmooMPα-I区域显示与目标物的最佳相互作用得分。使用无机磷酸盐底物和MESG测量PfPNP反应的比活值。由于对酪蛋白的蛋白水解活性和在约23 kDa处存在一个主要谱带,因此与BmooMPα-1对应的馏分在阳离子交换色谱步骤中被鉴定为馏分4。BmooMPα-1能够抑制W2恶性疟原虫的体外生长,IC50值为16.14μg/ mL。用Pep1 BM进行的虚拟筛选显示了两个PfPNP靶标结合区域,在相互作用界面处的ΔG值分别为-10.75 kcal / mol和-11.74 kcal / mol。与在不存在这种可能的抑制剂的情况下进行的测定相比,在存在Pep 1 BM的情况下观察到PfPNP的酶活性显着降低。BmooMPα-I在体外显示出抗W2恶性疟原虫的活性。通过计算机技术,Pep 1 BM被鉴定为对PfPNP的催化位点具有潜在的结合亲和力,并在体外抑制了其催化活性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号