当前位置:
X-MOL 学术
›
Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stability of the adeno-associated virus 8 reference standard material.
Gene Therapy ( IF 4.6 ) Pub Date : 2019-03-29 , DOI: 10.1038/s41434-019-0072-9 Magalie Penaud-Budloo 1 , Frédéric Broucque 1 , Katell Harrouet 1 , Mohammed Bouzelha 1 , Sylvie Saleun 1 , Sandy Douthe 1 , Susan D'Costa 2 , Sushma Ogram 2 , Oumeya Adjali 1 , Véronique Blouin 1 , Martin Lock 3 , Richard O Snyder 2 , Eduard Ayuso 1
Gene Therapy ( IF 4.6 ) Pub Date : 2019-03-29 , DOI: 10.1038/s41434-019-0072-9 Magalie Penaud-Budloo 1 , Frédéric Broucque 1 , Katell Harrouet 1 , Mohammed Bouzelha 1 , Sylvie Saleun 1 , Sandy Douthe 1 , Susan D'Costa 2 , Sushma Ogram 2 , Oumeya Adjali 1 , Véronique Blouin 1 , Martin Lock 3 , Richard O Snyder 2 , Eduard Ayuso 1
Affiliation
|
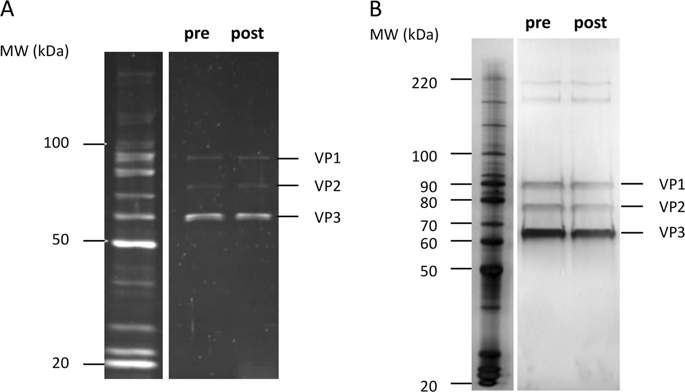
Adeno-associated virus (AAV) vectors are extensively used for gene therapy clinical trials. Accurate and standardized titration methods are essential for characterizing and dosing AAV-based drugs and thus to assess their safety and efficacy. To this end, the Reference Standard Materials (RSM) working group generated standards for AAV serotype 2 and serotype 8. The AAV8RSM (ATCC® VR-1816™) was deposited to the American Type Culture Collection in 2014 and is available to the scientific community. Here, three independent laboratories of the RSM working group provide stability data of the AAV8RSM 2 years after the initial characterization and after container relabeling performed at the ATCC. The AAV8RSM showed constant titers across experimental conditions: 1.48 ± 0.62 × 1012 vector genome (vg)/ml, 9.38 ± 11.4 × 108 infectious units (IU)/ml and 5.76 ± 2.39 × 1011 total particles (p)/ml as determined by qPCR, TCID50 and ELISA, respectively. Additionally, the AAV8RSM capsid protein integrity assessed by SDS-PAGE was equivalent to the original analyses. In conclusion, the AAV8RSM titers remained stable for two years under appropriate storage conditions ( <-70° C). The use of RSM is strongly recommended and endorsed by regulatory agencies to normalize laboratory internal controls and to provide accurate titration of AAV vectors lots.
中文翻译:

腺相关病毒8参考标准材料的稳定性。
腺相关病毒(AAV)载体被广泛用于基因治疗临床试验。准确和标准化的滴定方法对于表征和给药基于AAV的药物,从而评估其安全性和有效性至关重要。为此,参考标准材料(RSM)工作组生成了AAV血清型2和8血清型的标准。AAV8RSM(ATCC®VR-1816™)于2014年存放于美国典型培养物保藏中心,可供科学界使用。 。在此,RSM工作组的三个独立实验室提供了最初表征后2年和在ATCC进行容器重新贴标后2年内AAV8RSM的稳定性数据。AAV8RSM在整个实验条件下均显示恒定滴度:1.48±0.62×1012载体基因组(vg)/ml、9.38±11.4×108感染单位(IU)/ ml和5。分别通过qPCR,TCID50和ELISA测定的总颗粒(p)/ ml为76±2.39×1011。此外,通过SDS-PAGE评估的AAV8RSM衣壳蛋白完整性与原始分析相当。总之,AAV8RSM滴度在适当的储存条件下(<-70°C)保持稳定两年。强烈建议使用RSM,并得到监管机构的认可,以标准化实验室内部控制并提供准确的AAV载体滴定滴定。
更新日期:2019-11-18
中文翻译:

腺相关病毒8参考标准材料的稳定性。
腺相关病毒(AAV)载体被广泛用于基因治疗临床试验。准确和标准化的滴定方法对于表征和给药基于AAV的药物,从而评估其安全性和有效性至关重要。为此,参考标准材料(RSM)工作组生成了AAV血清型2和8血清型的标准。AAV8RSM(ATCC®VR-1816™)于2014年存放于美国典型培养物保藏中心,可供科学界使用。 。在此,RSM工作组的三个独立实验室提供了最初表征后2年和在ATCC进行容器重新贴标后2年内AAV8RSM的稳定性数据。AAV8RSM在整个实验条件下均显示恒定滴度:1.48±0.62×1012载体基因组(vg)/ml、9.38±11.4×108感染单位(IU)/ ml和5。分别通过qPCR,TCID50和ELISA测定的总颗粒(p)/ ml为76±2.39×1011。此外,通过SDS-PAGE评估的AAV8RSM衣壳蛋白完整性与原始分析相当。总之,AAV8RSM滴度在适当的储存条件下(<-70°C)保持稳定两年。强烈建议使用RSM,并得到监管机构的认可,以标准化实验室内部控制并提供准确的AAV载体滴定滴定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号