European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-03-26 , DOI: 10.1016/j.ejmech.2019.03.058 Yuanyuan Li , Silong Zhang , Jing Zhang , Zhiye Hu , Yuan Xiao , Jian Huang , Chune Dong , Shengtang Huang , Hai-Bing Zhou
|
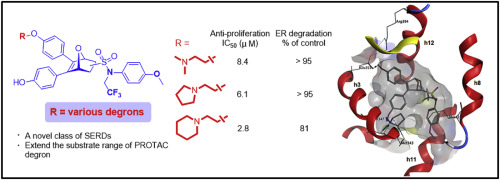
As the mutant estrogen receptor (ER) continues to be characterized, breast cancer is becoming increasingly difficult to cure when treated with hormone therapy. In this regard, a strategy to selectively and effectively degrade the ER might be an effective alternative to endocrine therapy for breast cancer. In a previous study, we identified a novel series of 7-oxabicyclo[2.2.1]heptene sulfonamide (OBHSA) compounds as full ER antagonists while lacking the prototypical ligand side chain that has been widely used to induce antagonism of ERα. Further crystal structure studies and phenotypic assays revealed that these compounds are selective estrogen receptor degraders (SERDs) with a new mechanism of action. However, from a drug discovery point of view, there still is room to improve the potency of these OBHSA compounds. In this study, we have developed new classes of SERDs that contain the OBHSA core structure and different side chains, e.g., basic side chains, long alkyl acid side chains, and glycerol ether side chains, to simply mimic the degrons of proteolysis targeting chimera (PROTAC) and then investigated the structure-activity relationships of these PROTAC-like hybrid compounds. These novel SERDs could effectively inhibit MCF-7 cell proliferation and demonstrated good ERα degradation efficacy. Among the SERDs, compounds 17d, 17e and 17g containing a basic side chain with a N-trifluoroethyl substituent and a para methoxyl group at the phenyl group of the sulfonamide turned out to be the best candidates for ER degraders. A further docking study of these compounds with ERα elucidates their structure-activity relationships, which provides guidance to design new PROTAC degrons targeting ER for breast cancer therapy. Lastly, easy modification of these PROTAC-like SERDs enables further fine-tuning of their pharmacokinetic properties, including oral availability.
中文翻译:

探索PROTAC degron候选物:具有不同侧链的OBHSA作为新型选择性雌激素受体降解剂(SERD)
随着突变雌激素受体(ER)的继续表征,用激素疗法治疗乳腺癌变得越来越困难。在这方面,选择性和有效降解ER的策略可能是乳腺癌内分泌治疗的有效替代方法。在先前的研究中,我们确定了一系列新型的7-氧杂双环[2.2.1]庚烯磺酰胺(OBHSA)化合物,作为完全的ER拮抗剂,但缺少已广泛用于诱导ERα拮抗作用的原型配体侧链。进一步的晶体结构研究和表型分析表明,这些化合物是具有新作用机制的选择性雌激素受体降解剂(SERD)。但是,从药物发现的角度来看,仍有提高这些药物效力的余地OBHSA化合物。在这项研究中,我们开发了新型的SERD,它们包含OBHSA核心结构和不同的侧链,例如碱性侧链,长烷基酸侧链和甘油醚侧链,以简单地模拟针对嵌合体的蛋白水解的地龙( PROTAC),然后研究了这些PROTAC样杂化化合物的构效关系。这些新颖的SERDs可以有效抑制MCF-7细胞增殖,并表现出良好的ERα降解功效。在SERD中,化合物17d,17e和17g含有带有N-三氟乙基取代基和对位的碱性侧链磺酰胺的苯基上的甲氧基被证明是ER降解剂的最佳候选者。这些化合物与ERα的进一步对接研究阐明了它们的构效关系,为设计靶向ER的新型PROTAC degrons用于乳腺癌治疗提供了指导。最后,对这些类似PROTAC的SERD进行轻松修饰,就可以进一步微调其药代动力学特性,包括口服有效性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号