Immunity ( IF 25.5 ) Pub Date : 2019-03-19 , DOI: 10.1016/j.immuni.2019.02.009 Xin Li , Meng Deng , Alex S. Petrucelli , Cheng Zhu , Jinyao Mo , Lu Zhang , Jason W. Tam , Pablo Ariel , Baoyu Zhao , Song Zhang , Hengming Ke , Pingwei Li , Nikolay V. Dokholyan , Joseph A. Duncan , Jenny P.-Y. Ting
|
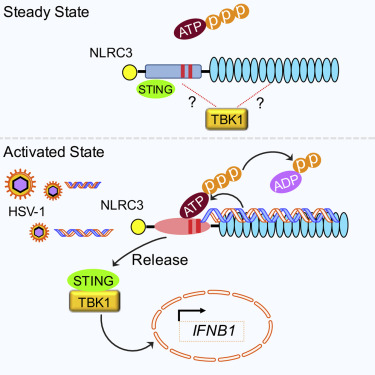
Immune suppression is a crucial component of immunoregulation and a subgroup of nucleotide-binding domain (NBD), leucine-rich repeat (LRR)-containing proteins (NLRs) attenuate innate immunity. How this inhibitory function is controlled is unknown. A key question is whether microbial ligands can regulate this inhibition. NLRC3 is a negative regulator that attenuates type I interferon (IFN-I) response by sequestering and attenuating stimulator of interferon genes (STING) activation. Here, we report that NLRC3 binds viral DNA and other nucleic acids through its LRR domain. DNA binding to NLRC3 increases its ATPase activity, and ATP-binding by NLRC3 diminishes its interaction with STING, thus licensing an IFN-I response. This work uncovers a mechanism wherein viral nucleic acid binding releases an inhibitory innate receptor from its target.
中文翻译:

与抑制性核酸传感器NLRC3结合的病毒DNA释放STING,这是激活I型干扰素的环状二核苷酸受体。
免疫抑制是免疫调节的重要组成部分,核苷酸结合结构域(NBD)的一个亚组,富含亮氨酸重复序列(LRR)的蛋白质(NLRs)减弱了先天免疫力。如何抑制这种抑制功能是未知的。一个关键问题是微生物配体是否可以调节这种抑制作用。NLRC3是一种负调节剂,可通过隔离和减弱干扰素基因激活(STING)的刺激物来减弱I型干扰素(IFN-I)的应答。在这里,我们报道NLRC3通过其LRR结构域结合病毒DNA和其他核酸。DNA与NLRC3的结合会增加其ATPase活性,而NLRC3与ATP的结合会减少其与STING的相互作用,从而获得IFN-I应答。这项工作揭示了一种机制,其中病毒核酸结合从其靶标释放抑制性先天受体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号