International Journal of Oral Science ( IF 10.8 ) Pub Date : 2019-03-18 , DOI: 10.1038/s41368-019-0046-1 Jianfei Zhang , Wenbin Zhang , Jiewen Dai , Xudong Wang , Steve Guofang Shen
|
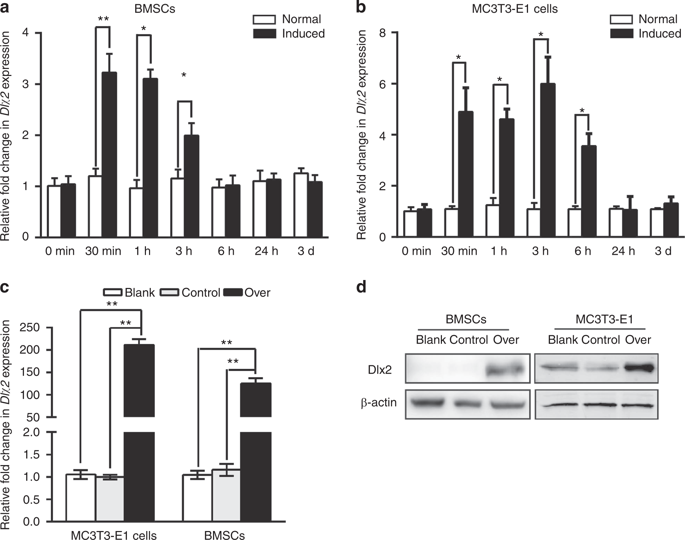
Genetic studies have revealed a critical role of Distal-homeobox (Dlx) genes in bone formation, and our previous study showed that Dlx2 overexpressing in neural crest cells leads to profound abnormalities of the craniofacial tissues. The aim of this study was to investigate the role and the underlying molecular mechanisms of Dlx2 in osteogenic differentiation of mouse bone marrow stromal cells (BMSCs) and pre-osteoblast MC3T3-E1 cells. Initially, we observed upregulation of Dlx2 during the early osteogenesis in BMSCs and MC3T3-E1 cells. Moreover, Dlx2 overexpression enhanced alkaline phosphatase (ALP) activity and extracellular matrix mineralization in BMSCs and MC3T3-E1 cell line. In addition, micro-CT of implanted tissues in nude mice confirmed that Dlx2 overexpression in BMSCs promoted bone formation in vivo. Unexpectedly, Dlx2 overexpression had little impact on the expression level of the pivotal osteogenic transcription factors Runx2, Dlx5, Msx2, and Osterix, but led to upregulation of Alp and Osteocalcin (OCN), both of which play critical roles in promoting osteoblast maturation. Importantly, luciferase analysis showed that Dlx2 overexpression stimulated both OCN and Alp promoter activity. Through chromatin-immunoprecipitation assay and site-directed mutagenesis analysis, we provide molecular evidence that Dlx2 transactivates OCN and Alp expression by directly binding to the Dlx2-response cis-acting elements in the promoter of the two genes. Based on these findings, we demonstrate that Dlx2 overexpression enhances osteogenic differentiation in vitro and accelerates bone formation in vivo via direct upregulation of the OCN and Alp gene, suggesting that Dlx2 plays a crucial role in osteogenic differentiation and bone formation.
中文翻译:

Dlx2的过表达通过直接上调骨钙素和Alp增强BMSCs和MC3T3-E1细胞的成骨分化
遗传学研究揭示了远侧同源盒(Dlx)基因在骨骼形成中的关键作用,而我们先前的研究表明Dlx2在神经c细胞中的过度表达会导致颅面组织的严重异常。这项研究的目的是调查Dlx2在小鼠骨髓基质细胞(BMSCs)和成骨细胞MC3T3-E1细胞成骨分化中的作用及其潜在的分子机制。最初,我们观察到BMSCs和MC3T3-E1细胞在早期成骨过程中Dlx2的上调。此外,Dlx2过表达增强了BMSCs和MC3T3-E1细胞系中的碱性磷酸酶(ALP)活性和细胞外基质矿化作用。此外,对裸鼠植入的组织进行显微CT证实,BMSC中D1x2的过表达促进了体内的骨形成。不料,DLX2表达对举足轻重的成骨转录因子的表达水平影响不大Runx2的,DLX5,MSX2和Osterix的,但导致的上调高山和骨钙素(OCN),两者在促进成骨细胞的成熟起到关键作用。重要的是,萤光素酶分析显示Dlx2过表达刺激了OCN和Alp启动子活性。通过染色质免疫沉淀测定和定点诱变分析,我们提供了分子证据,表明Dlx2通过直接结合两个基因启动子中的Dlx2反应顺式作用元件来激活OCN和Alp表达。基于这些发现,我们证明Dlx2的过表达增强了体外的成骨分化,并通过直接上调OCN和Alp基因而在体内加速了骨的形成,表明Dlx2在成骨的分化和骨形成中起着至关重要的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号