Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Critical role of the finger loop in arrestin binding to the receptors.
PLOS ONE ( IF 2.9 ) Pub Date : 2019-03-15 , DOI: 10.1371/journal.pone.0213792 Chen Zheng 1 , Jonas Tholen 2 , Vsevolod V Gurevich 1
PLOS ONE ( IF 2.9 ) Pub Date : 2019-03-15 , DOI: 10.1371/journal.pone.0213792 Chen Zheng 1 , Jonas Tholen 2 , Vsevolod V Gurevich 1
Affiliation
|
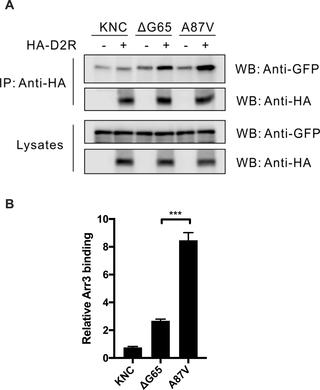
We tested the interactions with four different G protein-coupled receptors (GPCRs) of arrestin-3 mutants with substitutions in the four loops, three of which contact the receptor in the structure of the arrestin-1-rhodopsin complex. Point mutations in the loop at the distal tip of the N-domain (Glu157Ala), in the C-loop (Phe255Ala), back loop (Lys313Ala), and one of the mutations in the finger loop (Gly65Pro) had mild variable effects on receptor binding. In contrast, the deletion of Gly65 at the beginning of the finger loop reduced the binding to all GPCRs tested, with the binding to dopamine D2 receptor being affected most dramatically. Thus, the presence of a glycine at the beginning of the finger loop appears to be critical for the arrestin-receptor interaction.
中文翻译:

指环在抑制素与受体结合中的关键作用。
我们测试了抑制素3突变体与四个不同的G蛋白偶联受体(GPCR)的相互作用,在四个环中有取代,其中三个与抑制素1视紫红质复合物的结构中的受体接触。N域远端(Glu157Ala),C环(Phe255Ala),反向环(Lys313Ala)环中的点突变以及手指环(Gly65Pro)中的一种突变对受体结合。相反,在指环开始处缺失Gly65降低了与所有测试GPCR的结合,与多巴胺D2受体的结合受到的影响最大。因此,在指环开始时甘氨酸的存在对于抑制素-受体相互作用似乎至关重要。
更新日期:2019-03-17
中文翻译:

指环在抑制素与受体结合中的关键作用。
我们测试了抑制素3突变体与四个不同的G蛋白偶联受体(GPCR)的相互作用,在四个环中有取代,其中三个与抑制素1视紫红质复合物的结构中的受体接触。N域远端(Glu157Ala),C环(Phe255Ala),反向环(Lys313Ala)环中的点突变以及手指环(Gly65Pro)中的一种突变对受体结合。相反,在指环开始处缺失Gly65降低了与所有测试GPCR的结合,与多巴胺D2受体的结合受到的影响最大。因此,在指环开始时甘氨酸的存在对于抑制素-受体相互作用似乎至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号