Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An integrated analysis of safety and tolerability of etelcalcetide in patients receiving hemodialysis with secondary hyperparathyroidism.
PLOS ONE ( IF 2.9 ) Pub Date : 2019-03-15 , DOI: 10.1371/journal.pone.0213774 Geoffrey A Block 1 , Glenn M Chertow 2 , John T Sullivan 3 , Hongjie Deng 3 , Omar Mather 3 , Holly Tomlin 3 , Michael Serenko 3
PLOS ONE ( IF 2.9 ) Pub Date : 2019-03-15 , DOI: 10.1371/journal.pone.0213774 Geoffrey A Block 1 , Glenn M Chertow 2 , John T Sullivan 3 , Hongjie Deng 3 , Omar Mather 3 , Holly Tomlin 3 , Michael Serenko 3
Affiliation
|
BACKGROUND
Calcimimetics have been shown to be effective and safe therapies for the treatment of secondary hyperparathyroidism (sHPT), a serious complication of disordered mineral metabolism associated with dialysis-dependent chronic kidney disease. Etelcalcetide, a recently approved intravenous calcimimetic, reduces serum parathyroid hormone (PTH), calcium, phosphorus, and fibroblast growth factor-23 concentrations. Here we report the first integrated safety profile of etelcalcetide using pooled data from five pivotal clinical trials.
METHODS
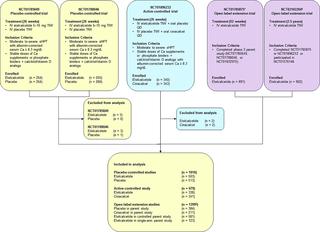
This analysis included data from patients receiving hemodialysis with moderate to severe sHPT enrolled in two randomized, placebo-controlled trials; a randomized active-controlled (with cinacalcet) trial; and two single-arm, open-label extension trials. Patients initially received etelcalcetide intravenously 5 mg three times weekly (TIW) after hemodialysis; with potential dose increases of 2.5 or 5 mg at 4-week intervals to a maximum dose of 15 mg TIW, depending on serum PTH and calcium levels. The nature, frequency, and severity of treatment-emergent adverse events (AEs) and changes in laboratory parameters were assessed.
RESULTS
Overall, we evaluated 1023 patients from the placebo-controlled trials, 683 from the active-controlled trial, and 1299 from open-label extensions. The frequency and nature of common treatment-emergent AEs reported for the etelcalcetide arm were consistent among the placebo-controlled and active-controlled trials. The most common AEs were those related to mineral metabolism (decreased blood calcium, hypophosphatemia, muscle spasms) or gastrointestinal abnormalities (diarrhea, nausea, vomiting). Hypocalcemia leading to discontinuation of either calcimimetic was experienced in ≤ 1% of patients.
CONCLUSIONS
This integrated safety assessment of etelcalcetide across placebo- and active-controlled trials showed an overall favorable risk/benefit profile, with safety similar to that of cinacalcet. Consistent with its mechanism of action, the most important risks associated with etelcalcetide were serum calcium reductions and hypocalcemia-related AEs; no new safety findings were identified in the pooled long-term extension trials.
中文翻译:

接受血液透析合并继发性甲状旁腺功能亢进患者的依卡西肽安全性和耐受性综合分析。
背景技术拟钙剂已被证明是治疗继发性甲状旁腺功能亢进症(sHPT)的有效和安全的疗法,后者是与透析相关的慢性肾脏疾病相关的矿物质代谢紊乱的严重并发症。Etelcalcetide是最近被批准的静脉拟钙剂,可降低血清甲状旁腺激素(PTH),钙,磷和成纤维细胞生长因子23的浓度。在这里,我们使用来自五个关键临床试验的汇总数据报告了依替卡西肽的第一个综合安全性分析。方法该分析包括两项随机,安慰剂对照试验中接受中度至重度sHPT的血液透析患者的数据。一项随机的主动对照(使用西那卡塞)试验;和两项单臂开放标签扩展试验。患者最初接受血液透析后每周三次(TIW)静脉滴注ecalccalcetide 5 mg;每4周间隔增加2.5或5 mg的剂量,最大剂量为15 mg TIW,具体取决于血清PTH和钙水平。评估了治疗紧急不良事件(AE)的性质,频率和严重性以及实验室参数的变化。结果总体而言,我们评估了安慰剂对照试验的1023例患者,活性对照试验的683例和开放标签扩展试验的1299例患者。在安慰剂对照试验和活性药物对照试验中,报告给依替卡塞肽治疗的常见紧急治疗性不良事件的发生频率和性质一致。最常见的AE是与矿物质代谢有关的(血液钙减少,低血磷,肌肉痉挛)或胃肠道异常(腹泻,恶心,呕吐)。导致≤1%的患者发生低钙血症,导致任何一种拟钙剂的停用。结论在安慰剂对照和主动对照试验中,对依卡西肽的综合安全性评估显示总体良好的风险/获益概况,其安全性与西那卡塞相似。与它的作用机理一致,与依替卡列肽相关的最重要的风险是血清钙减少和与低钙血症相关的AE。在汇总的长期延伸试验中未发现新的安全性发现。结论在安慰剂对照和主动对照试验中,对依卡西肽的综合安全性评估显示总体良好的风险/获益概况,其安全性与西那卡塞相似。与它的作用机理一致,与依卡他肽相关的最重要的风险是血清钙减少和与低钙血症相关的AE。在汇总的长期延伸试验中未发现新的安全性发现。结论在安慰剂对照和主动对照试验中,对依卡西肽的综合安全性评估显示总体良好的风险/获益概况,其安全性与西那卡塞相似。与它的作用机理一致,与依替卡列肽相关的最重要的风险是血清钙减少和与低钙血症相关的AE。在汇总的长期延伸试验中未发现新的安全性发现。
更新日期:2019-03-17
中文翻译:

接受血液透析合并继发性甲状旁腺功能亢进患者的依卡西肽安全性和耐受性综合分析。
背景技术拟钙剂已被证明是治疗继发性甲状旁腺功能亢进症(sHPT)的有效和安全的疗法,后者是与透析相关的慢性肾脏疾病相关的矿物质代谢紊乱的严重并发症。Etelcalcetide是最近被批准的静脉拟钙剂,可降低血清甲状旁腺激素(PTH),钙,磷和成纤维细胞生长因子23的浓度。在这里,我们使用来自五个关键临床试验的汇总数据报告了依替卡西肽的第一个综合安全性分析。方法该分析包括两项随机,安慰剂对照试验中接受中度至重度sHPT的血液透析患者的数据。一项随机的主动对照(使用西那卡塞)试验;和两项单臂开放标签扩展试验。患者最初接受血液透析后每周三次(TIW)静脉滴注ecalccalcetide 5 mg;每4周间隔增加2.5或5 mg的剂量,最大剂量为15 mg TIW,具体取决于血清PTH和钙水平。评估了治疗紧急不良事件(AE)的性质,频率和严重性以及实验室参数的变化。结果总体而言,我们评估了安慰剂对照试验的1023例患者,活性对照试验的683例和开放标签扩展试验的1299例患者。在安慰剂对照试验和活性药物对照试验中,报告给依替卡塞肽治疗的常见紧急治疗性不良事件的发生频率和性质一致。最常见的AE是与矿物质代谢有关的(血液钙减少,低血磷,肌肉痉挛)或胃肠道异常(腹泻,恶心,呕吐)。导致≤1%的患者发生低钙血症,导致任何一种拟钙剂的停用。结论在安慰剂对照和主动对照试验中,对依卡西肽的综合安全性评估显示总体良好的风险/获益概况,其安全性与西那卡塞相似。与它的作用机理一致,与依替卡列肽相关的最重要的风险是血清钙减少和与低钙血症相关的AE。在汇总的长期延伸试验中未发现新的安全性发现。结论在安慰剂对照和主动对照试验中,对依卡西肽的综合安全性评估显示总体良好的风险/获益概况,其安全性与西那卡塞相似。与它的作用机理一致,与依卡他肽相关的最重要的风险是血清钙减少和与低钙血症相关的AE。在汇总的长期延伸试验中未发现新的安全性发现。结论在安慰剂对照和主动对照试验中,对依卡西肽的综合安全性评估显示总体良好的风险/获益概况,其安全性与西那卡塞相似。与它的作用机理一致,与依替卡列肽相关的最重要的风险是血清钙减少和与低钙血症相关的AE。在汇总的长期延伸试验中未发现新的安全性发现。










































 京公网安备 11010802027423号
京公网安备 11010802027423号