当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and evaluation of novel heteroaryldihydropyrimidine derivatives as non-nucleoside hepatitis B virus inhibitors by exploring the solvent-exposed region.
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2019-03-01 , DOI: 10.1111/cbdd.13512 Ji Yu 1 , Haiyong Jia 2 , Xiaowei Guo 3 , Samuel Desta 1 , Shuo Zhang 1 , Jian Zhang 1 , Xiao Ding 1 , Xiaohong Liang 3 , Xinyong Liu 1 , Peng Zhan 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2019-03-01 , DOI: 10.1111/cbdd.13512 Ji Yu 1 , Haiyong Jia 2 , Xiaowei Guo 3 , Samuel Desta 1 , Shuo Zhang 1 , Jian Zhang 1 , Xiao Ding 1 , Xiaohong Liang 3 , Xinyong Liu 1 , Peng Zhan 1
Affiliation
|
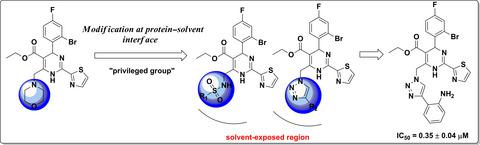
In continuation of our efforts toward the discovery of potent non-nucleoside hepatitis B virus (HBV) inhibitors with novel structures, we have explored the solvent-exposed protein region of heteroaryldihydropyrimidine derivatives. Herein, the morpholine ring of GLS4 was replaced with substituted sulfonamides and triazoles to generate novel non-nucleoside HBV inhibitors with desirable potency. In in vitro biological evaluation, several derivatives showed good anti-HBV DNA replication activity compared to lamivudine. In particular, compound II-1 displayed the most potent activity against HBV DNA replication (IC50 = 0.35 ± 0.04 μM). The preliminary structure-activity relationships of the new compounds were summarized, which may help in discovering more potent anti-HBV agents via rational drug design.
中文翻译:

通过探索溶剂暴露区域,设计,合成和评估作为非核苷乙型肝炎病毒抑制剂的新型杂芳基二氢嘧啶衍生物。
在继续努力发现具有新颖结构的有效非核苷乙型肝炎病毒(HBV)抑制剂的过程中,我们探索了杂芳基二氢嘧啶衍生物的溶剂暴露蛋白区域。在本文中,将GLS4的吗啉环替换为取代的磺酰胺和三唑,以产生具有所需效价的新型非核苷HBV抑制剂。在体外生物学评估中,与拉米夫定相比,几种衍生物显示出良好的抗HBV DNA复制活性。特别是,化合物II-1对HBV DNA复制表现出最有效的活性(IC50 = 0.35±0.04μM)。总结了新化合物的初步构效关系,这可能有助于通过合理的药物设计发现更有效的抗HBV药物。
更新日期:2019-03-01
中文翻译:

通过探索溶剂暴露区域,设计,合成和评估作为非核苷乙型肝炎病毒抑制剂的新型杂芳基二氢嘧啶衍生物。
在继续努力发现具有新颖结构的有效非核苷乙型肝炎病毒(HBV)抑制剂的过程中,我们探索了杂芳基二氢嘧啶衍生物的溶剂暴露蛋白区域。在本文中,将GLS4的吗啉环替换为取代的磺酰胺和三唑,以产生具有所需效价的新型非核苷HBV抑制剂。在体外生物学评估中,与拉米夫定相比,几种衍生物显示出良好的抗HBV DNA复制活性。特别是,化合物II-1对HBV DNA复制表现出最有效的活性(IC50 = 0.35±0.04μM)。总结了新化合物的初步构效关系,这可能有助于通过合理的药物设计发现更有效的抗HBV药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号