Journal of Molecular Catalysis A: Chemical Pub Date : 2016-07-28 , DOI: 10.1016/j.molcata.2016.07.050 Tiago A. Fernandes , Vânia André , Alexander M. Kirillov , Marina V. Kirillova
|
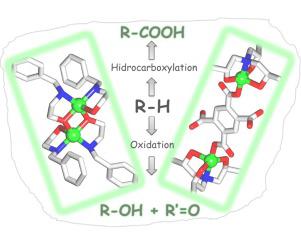
N-benzylethanolamine (Hbea) and triisopropanolamine (H3tipa) were applied as unexplored aminoalcohol N,O-building blocks for the self-assembly generation of two novel dicopper(II) compounds, [Cu2(μ-bea)2(Hbea)2](NO3)2 (1) and [Cu2(H3tipa)2(μ-pma)]·7H2O (2) {H4pma = pyromellitic acid}. These were isolated as stable and aqua-soluble microcrystalline products and were fully characterized by IR spectroscopy, ESI–MS(±), and single-crystal X-ray diffraction, the latter revealing distinct Cu2 cores containing the five-coordinate copper(II) centers with the {CuN2O3} or {CuNO4} environments. Compounds 1 and 2 were used as homogeneous catalysts for the mild oxidation of C5–C8 cycloalkanes to give the corresponding cyclic alcohols and ketones in up to 23% overall yields based on cycloalkane. The reactions proceed in aqueous acetonitrile medium at 50 °C using H2O2 as an oxidant. The effects of different reaction conditions were studied, including the type and loading of catalyst, amount and kind of acid promoter, and water concentration. Despite the fact that different acids (HNO3, H2SO4, HCl, or CF3COOH) promote the oxidation of alkanes, the reaction is exceptionally fast in the presence of a catalytic amount of HCl, resulting in the TOF values of up to 430 h−1. Although water typically strongly inhibits alkane oxidations due to the reduction of H2O2 concentration and lowering of the alkane solubility, in the systems comprising 1 and 2 we observed a significant growth (up to 5-fold) of an initial reaction rate in the cyclohexane oxidation on increasing the amount of H2O in the reaction mixture. The bond-, regio- and stereo-selectivity parameters were investigated in oxidation of different linear, branched, and cyclic alkane substrates. Both compounds 1 and 2 also catalyze the hydrocarboxylation of C5–C8 cycloalkanes, by CO, K2S2O8, and H2O in a water/acetonitrile medium at 60 °C, to give the corresponding cycloalkanecarboxylic acids in up to 38% yields based on cycloalkanes.
中文翻译:

新型双铜(II)氨基醇驱动核催化环烷烃的轻度均相氧化和加氢羧化反应
N-苄基乙醇胺(Hbea)和三异丙醇胺(H 3 tipa)被用作未开发的氨基醇N,O-砌块,用于自组装生成两种新型双铜(II)化合物[Cu 2(μ-bea)2(Hbea )2 ](NO 3)2(1)和[Cu 2(H 3 tipa)2(μ-pma)]·7H 2 O(2){H 4pma =均苯四酸}。它们被分离为稳定且水溶性的微晶产物,并通过红外光谱,ESI–MS(±)和单晶X射线衍射进行了充分表征,后者显示出含有五配位铜(II)的独特Cu 2核)以{CuN 2 O 3 }或{CuNO 4 }环境为中心。化合物1和2用作C 5 – C 8环烷烃的轻度氧化的均相催化剂,以环烷烃为基准,以高达23%的总收率得到相应的环状醇和酮。使用H 2 O在50°C的乙腈水溶液中进行反应2作为氧化剂。研究了不同反应条件的影响,包括催化剂的类型和负载量,助酸剂的量和种类以及水的浓度。尽管存在不同的酸(HNO 3,H 2 SO 4,HCl或CF 3 COOH)促进烷烃氧化的事实,但在催化量的HCl存在下,反应异常快,导致TOF值升高。至430h -1。尽管由于H 2 O 2浓度的降低和烷烃溶解度的降低,水通常会强烈抑制烷烃的氧化,但在包含1和2的系统中我们观察到,随着反应混合物中H 2 O量的增加,环己烷氧化反应的初始反应速率显着提高(最高5倍)。在不同的线性,支链和环状烷烃底物的氧化过程中研究了键,区域和立体选择性参数。化合物1和2还在60°C的水/乙腈介质中通过CO,K 2 S 2 O 8和H 2 O催化C 5 –C 8环烷烃的加氢羧化反应,从而生成相应的环烷羧酸。以环烷烃计,收率为38%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号