Journal of Molecular Catalysis A: Chemical Pub Date : 2016-08-16 , DOI: 10.1016/j.molcata.2016.08.014 Ekaterina M. Titova , S.M. Wahidur Rahaman , Elena S. Shubina , Rinaldo Poli , Natalia V. Belkova , Eric Manoury
|
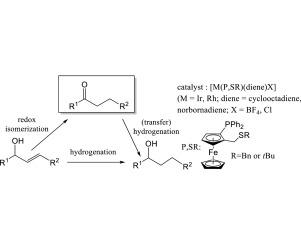
Complexes [M(P,SR)(diene)X] where (P,SR) = CpFe[1,2-C5H3(PPh2)(CH2SR)] (M = Ir, R = tBu or Bn diene = cod, X = Cl; M = Rh, diene = cod or nbd; X = BF4 or Cl) were used as precatalysts for the redox isomerization of various allylic alcohols (7a–e) to the corresponding saturated ketones (8a–e) and or hydrogenation to the saturated alcohol (9a–e). In optimization studies using 1-phenyl-2-propen-1-ol (7a) in THF and in iPrOH/MeONa, the only observed product was the saturated alcohol 1-phenyl-1-propanol (9a) when working under a 30 bar H2 pressure, but activation for only 1 min under H2 pressure and then continuation under 1 bar of H2 or Ar led to increasing amounts of the allylic isomerization product propiophenone (8a). Continued reaction under H2 converted (8a) into (9a). The Rh precatalysts were more active than the Ir analogues. For the rhodium precatalysts (3) and (4), the redox isomerization reaction could be carried out after precatalyst activation in iPrOH/MeONa under Ar at 82 °C (without H2) with complete conversion in 1 h (1% catalyst loading). However, longer reaction times resulted in slow transfer hydrogenation of (8a) leading to (9a) with low enantiomeric excess. Extension of the H2-free activation of the Rh precatalysts in iPrOH to other allylic alcohol substrates (7b–d) yielded the corresponding ketones with good to excellent yields and excellent chemoselectivities under appropriate conditions.
中文翻译:

带有二茂铁膦-硫醚配体的铑和铱配合物催化烯丙基醇的氧化还原异构化
配合物[M(P,SR)(diene)X],其中(P,SR)= CpFe [1,2-C 5 H 3(PPh 2)(CH 2 SR)](M = Ir,R = tBu或Bn二烯= cod,X = Cl; M = Rh,二烯= cod或nbd; X = BF 4或Cl)用作各种烯丙基醇(7a–e)氧化还原为相应饱和酮(8a –e)的预催化剂e)和/或加氢成饱和醇(9a–e)。在THF和iPrOH / MeONa中使用1-苯基-2-丙-1-醇(7a)进行的优化研究中,观察到的唯一产物是在30°C下工作的饱和醇1-苯基-1-丙醇(9 a)。 H 2栏压力,但在H 2压力下仅活化1分钟,然后在1 bar H 2或Ar下持续进行会导致烯丙基异构化产物丙苯酮(8a)的量增加。在H 2下继续反应,将(8a)转化为(9a)。Rh预催化剂比Ir类似物更具活性。对于铑预催化剂(3)和(4),在iPrOH / MeONa中在82°C(无H 2)下在iPrOH / MeONa中将预催化剂活化后,可以在1小时内完全转化(1%催化剂负载)下进行氧化还原异构化反应。。但是,较长的反应时间会导致(8a)导致对映体过量低的(9a)。将iPrOH中Rh预催化剂的无H 2活化作用扩展到其他烯丙基醇底物(7b–d),可以得到相应的酮,在适当的条件下,酮具有良好或优异的产率和优异的化学选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号