Journal of Molecular Catalysis A: Chemical Pub Date : 2016-07-07 , DOI: 10.1016/j.molcata.2016.07.012 Oleg V. Khazipov , Denys V. Nykytenko , Tatyana V. Krasnyakova , Alexander N. Vdovichenko , Dario A. Fuentes Frias , Serge A. Mitchenko
|
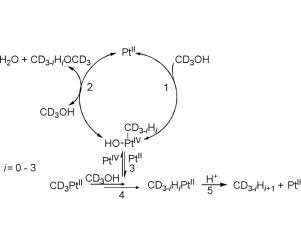
A novel catalytic reaction of alcohol etherification in the system ROH − PtCl42− ‐ PtCl62− was found. Methanol easily transforms into dimethyl ether in the presence of catalytic amounts of PtII chloro complexes at 70 °C. Under the same conditions reaction of ethanol affords diethyl ether (catalytic) and π-ethylene PtII complex (stoichiometric). The reactions are accompanied by multiple H/D exchange, which is indicative of intermediacy of corresponding alkyl platinum derivatives. The plausible reaction mechanism involves oxidative addition of alcohol forming intermediate alkyl platinum(IV) derivative followed by decomposition of it via reductive elimination step under the action of alcohol giving the ether and regenerating catalyst. In the case of ethyl alcohol reaction, β-hydrogen abstraction from the intermediate Pt-ethyl species yields π-ethylene platinum(II) complex. Although it seems that the reaction does not involve the initial breaking of CH bonds of an alcohol, this system can be regarded as a model for studying of some peculiarities of Shilov chemistry, in particular, of isotope scrambling mechanisms in Shilov alkane activation.
In contrast to reactions of dimethyl and diethyl ethers formation, tert-butyl ethers formation in CD3OH/t-BuOH medium is catalyzed by PtIV chloro complexes also and is not accompanied by isotope scrambling. These observations argue against intermediacy of alkyl platinum derivatives suggesting that acid-catalyzed mechanism operates in tert-butyl alcohol etherification.
中文翻译:

Shilov系统中醇的催化醚化:
发现了在ROH-PtCl 4 2 −-PtCl 6 2−体系中醇醚化的新型催化反应。在70℃催化量的Pt II氯配合物存在下,甲醇容易转化为二甲醚。在相同条件下,乙醇反应得到乙醚(催化)和π-乙烯Pt II配合物(化学计量)。反应伴随多次H / D交换,这指示相应的烷基铂衍生物的中间性。可能的反应机理包括将醇氧化加成形成中间体烷基铂(IV)衍生物,然后通过在醇的作用下进行还原消除步骤,得到醚并再生催化剂。在乙醇反应的情况下,从中间的Pt-乙基物质中提取β-氢会生成π-乙烯铂(II)配合物。尽管该反应似乎不涉及醇的C H键的初始断裂,但该系统可被视为研究Shilov化学的某些特性,特别是Shilov烷烃活化过程中同位素加扰机理的模型。
与二甲基和二乙醚形成的反应相反,CD 3 OH / t -BuOH介质中叔丁基醚的形成也受Pt IV氯配合物的催化,并且不伴有同位素加扰。这些观察结果反对烷基铂衍生物的中间体,表明酸催化的机理在叔丁醇醚化中起作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号