Journal of Molecular Catalysis A: Chemical Pub Date : 2016-07-26 , DOI: 10.1016/j.molcata.2016.07.044 Fernanda Parra da Silva , Renato V. Gonçalves , Liane M. Rossi
|
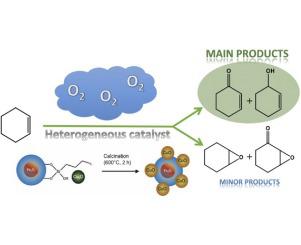
Magnetically recoverable copper oxide catalysts prepared by sol-immobilization method exhibited interesting properties for the allylic oxidation of cyclohexene with molecular oxygen as the sole oxidant. The catalysts were prepared by immobilization of pre-synthesized PVA (polyvinyl alcohol)-stabilized Cu2O nanoparticles (NPs) on a magnetically recoverable support; the catalyst was further oxidized to CuO NPs after calcination at 600 °C. Both catalysts can selectively oxidize cyclohexene through allylic oxidation to give 2-cyclohexene-1-one as the main product, but CuO was identified as the most active species providing 90% cyclohexene conversion and 96% selectivity for allylic products under 100 °C and 4 bar pressure of O2 for 6 h of reaction time. The catalysts were magnetically recovered without metal leaching and could be reused in at least six consecutive runs.
中文翻译:

磁性可回收的氧化铜催化剂,用于环己烯的好氧烯丙基氧化
通过溶胶固定化方法制备的可磁回收的氧化铜催化剂对于环己烯以分子氧作为唯一氧化剂的烯丙基氧化表现出令人感兴趣的性能。通过将预合成的PVA(聚乙烯醇)稳定化的Cu 2 O纳米颗粒(NPs)固定在可磁性回收的载体上来制备催化剂。在600°C煅烧后,该催化剂被进一步氧化为CuO NPs。两种催化剂都可以通过烯丙基氧化选择性氧化环己烯,以产生2-环己烯-1-酮为主要产物,但是CuO被认为是最具活性的物质,在100°C和4下,对烯丙基产物的转化率为90%,选择性为96%。 O 2的巴压反应时间为6小时。催化剂经过磁性回收,没有金属浸出,可以连续至少六次重复使用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号