Journal of Molecular Catalysis A: Chemical Pub Date : 2016-09-15 , DOI: 10.1016/j.molcata.2016.09.020 Claudia Rodrigues , Fabio G. Delolo , Jakob Norinder , Armin Börner , André L. Bogado , Alzir A. Batista
|
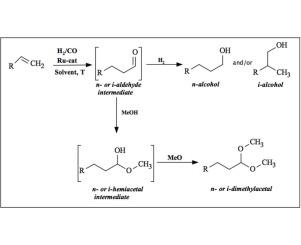
In this work, the catalytic activity of ruthenium II and III complexes containing chloride, pyridine, phosphine and CO ligands was investigated in the hydroformylation – hydrogenation and hydroformylation – acetalization reactions. The complexes mer-[RuCl3(dppb)(H2O)](1), mer-[RuCl3(dppb)(4-Vpy)](2), mer-[RuCl3(dppb)(4-tBupy)](3), mer-[RuCl3(dppb)(py)](4), mer-[RuCl3(dppb)(4-Phpy)](5), mer-[RuCl3(dppb)(4-Mepy)](6), cis-[RuCl2(CO)2(dppb)](7), trans-[RuCl2(CO)2(dppb)](8), RuCl3·xH2O(9), [RuCl2(PPh3)3](10) and [RuCl2(PPh3)2(dppb)](11) were used as supplied or synthesized as previously described in the literature {Where PPh3 = triphenylphosphine, dppb = 1,4-bis(diphenylphosphino)butane, py = pyridine, 4-Mepy = 4-methylpyridine, 4-Vpy = 4-vinylpyridine, 4-tBupy = 4-tert-butylpyridine and 4-Phpy = 4-phenylpyridine}. These complexes were used as a pre-catalysts in a hydroformylation catalytic system to produce CC, C
O and C
O bonds, where 1-decene resulted in a formation of respective alcohol and dimethyl acetals. Several reactions were performed in order to find the best reaction conditions presenting the best conversion (64% after 24 h). The 1-decene was also used as a substrate in two type tandem reactions labeled as: hydroformylation – hydrogenation (HH) and hydroformylation – acetalization (HA) reactions. The relationship between Ru – catalyst/substrate was 1:100, without free ligands or additives, in a controlled temperature and pressure. All the products of catalytic reactions HH and HA were analyzed by CG-FID with good yields.
中文翻译:

钌配合物催化的加氢甲酰化-加氢和加氢甲酰化-缩醛化反应
在这项工作中,在加氢甲酰化-加氢和加氢甲酰化-缩醛化反应中研究了含氯,吡啶,膦和CO配体的钌II和III配合物的催化活性。络合物可销将[RuCl 3(DPPB)(H 2 O)] (1) ,可销将[RuCl 3(DPPB)(4-VPY)] (2) ,可销将[RuCl 3(DPPB)(4-吨Bupy)] (3),mer- [RuCl 3(dppb)(py)] (4),mer- [RuCl 3(dppb)(4-Phpy)] (5),mer- [RuCl 3(dppb)(4-Mepy)] (6),顺式[RuCl 2(CO)2(dppb)] (7),反式[RuCl 2(CO)2(dppb)] (8 ),RuCl 3 ·xH 2 O (9),[RuCl 2(PPh 3)3 ] (10)和[RuCl 2(PPh 3)2(dppb)] (11)的使用或合成方法如前所述。文献{凡PPh 3 =三苯基膦,dppb = 1,4-双(二苯基膦基)丁烷,py =吡啶,4-Mepy = 4-甲基吡啶,4-Vpy = 4-乙烯基吡啶,4- t Bupy = 4-叔丁基吡啶和4-Phpy = 4-苯基吡啶}。这些配合物在加氢甲酰化催化体系中用作预催化剂,以生产C C,C
O和C
O键,其中1-癸烯导致分别形成醇和二甲基乙缩醛。为了找到表现出最佳转化率的最佳反应条件(24小时后为64%),进行了几次反应。1-癸烯还用作两种类型的串联反应的底物,分别标记为:加氢甲酰化–氢化(HH)和加氢甲酰化–缩醛化(HA)反应。在受控的温度和压力下,Ru-催化剂/底物之间的关系为1:100,没有游离的配体或添加剂。用CG-FID分析了催化反应HH和HA的所有产物,收率很高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号